Early Multidrug Treatment of SARS-Cov-2 (COVID-19) and Decreased Case Fatality Rates in Honduras
Early Multidrug, L Zeng, Sierra Hoffman, Valerio Pascua, Mccullough Pa, MD, MBA Sidney Ontai
Epidemiology International Journal, doi:10.23880/eij-16000217
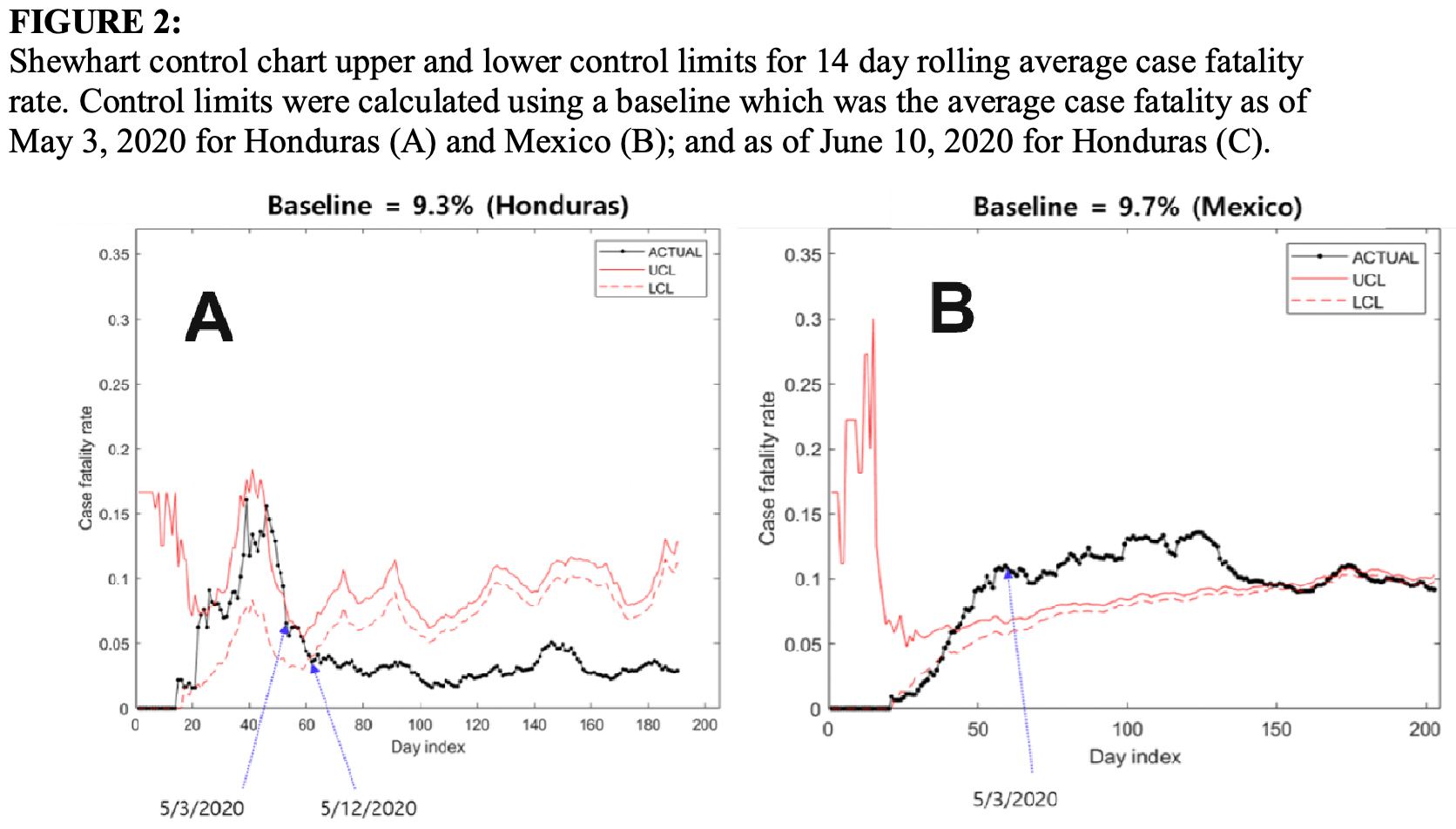
Introduction: Within 2 months of first detection of SARS-CoV-2 in Honduras, its government promoted nationwide implementation of multidrug COVID-19 inpatient and outpatient protocols. This was associated with a case fatality rate decrease from 9.33% to 2.97%. No decrease was seen in Mexico, a similar Latin-American country that did not introduce multidrug treatment protocols at that time. Objective: The primary objective of the study was to use statistical process control to assess the likelihood that the decrease in case fatality rate in Honduras was due to chance, using Mexico as a control country. Methods: Fourteen day running average COVID-19 case fatality rates in Mexico and Honduras were used to create Shewhart control charts during the first 6 months of the epidemic. The date of implementation in Honduras of the inpatient and outpatient multidrug COVID-19 protocols were plotted on control charts, with a Mexican COVID-19 case fatality control chart as a comparison.
Results: The case fatality rate for COVID-19 in Honduras dropped below the lower control limit 9 days after implementation of an inpatient multidrug inpatient protocol, from an average 9.33% case fatality rate to 5.01%. The Honduran COVID-19 case fatality rate again dropped below the 5.01% control chart limit 17 days after implementation of an outpatient multidrug protocol, to an average 2.97%, suggesting statistically significant anomalies. No control limit anomalies were seen during the same period in neighbouring Mexico.
Conclusion: There was an association between both inpatient and outpatient multidrug treatment of COVID-19 and decreased fatality rates in Honduras.
References
Bryant, Lawrie, Dowswell, Fordham, Mitchell, Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Metaanalysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am J Ther
Choi, Bhat, Velasquez, Welch, Coronavirus in New York City
Horby, Lim, Emberson, Mafham, Bell, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Lemos, Do, Santo, Salvetti, Gilo et al., Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID), Thromb Res
Mariette, Hermine, Tharaux, Rigon, Steg, Effectiveness of Tocilizumab in Patients Hospitalized With COVID-19: A Follow-up of the Corimuno-Toci-1 Randomized Clinical Trial, JAMA Intern Med
Perla, Provost, Parry, Little, Provost, Understanding variation in reported covid-19 death with a novel Shewhart chart application, Int J Qual Health Care
Sandhu, Tieng, Chilimuri, Franchin, A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection, Canadian Journal of Infectious Diseases and Medical Microbiology
Tardif, Bouabdallaoui, Allier, Gaudet, Shah, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Resp Med
Thor, Lundberg, Ask, Olsson, Carli, Application of statistical process control in healthcare improvement: systematic review, Qual Saf Health Care
DOI record:
{
"DOI": "10.23880/eij-16000217",
"ISSN": [
"2639-2038"
],
"URL": "http://dx.doi.org/10.23880/eij-16000217",
"author": [
{
"affiliation": [],
"family": "Ontai",
"given": "Sidney",
"sequence": "first"
}
],
"container-title": "Epidemiology International Journal",
"container-title-short": "EIJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
6
]
],
"date-time": "2022-08-06T07:11:30Z",
"timestamp": 1659769890000
},
"deposited": {
"date-parts": [
[
2022,
8,
6
]
],
"date-time": "2022-08-06T07:11:32Z",
"timestamp": 1659769892000
},
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:54:26Z",
"timestamp": 1714524866319
},
"is-referenced-by-count": 2,
"issue": "1",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022
]
]
}
},
"member": "9969",
"original-title": [
"Early Multidrug Treatment of SARS-Cov-2 (COVID-19) and Decreased Case Fatality Rates in Honduras"
],
"prefix": "10.23880",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"publisher": "Medwin Publishers",
"reference-count": 0,
"references-count": 0,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.07.21.21260223",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://medwinpublishers.com/EIJ/early-multidrug-treatment-of-sars-cov-2-covid-19-and-decreased-case-fatality-rates-in-honduras.pdf"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Early Multidrug Treatment of SARS-Cov-2 (COVID-19) and Decreased Case Fatality Rates in Honduras",
"type": "journal-article",
"volume": "6"
}