Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19
PhD Igor H Murai, PhD Alan L Fernandes, MSc Lucas P Sales, BSc Ana J Pinto, PhD Karla F Goessler, MD Camila S C Duran, MD Carla B R Silva, MD André S Franco, MD, MSc Marina B Macedo, MD Henrique H H Dalmolin, MD Janaina Baggio, MD Guilherme G M Balbi, PhD Bruna Z Reis, MD, PhD Leila Antonangelo, PhD; Valeria F Caparbo, PhD; Bruno Gualano, MD Rosa M R Pereira
JAMA, doi:10.1001/jama.2020.26848
IMPORTANCE The efficacy of vitamin D 3 supplementation in coronavirus disease 2019 (COVID-19) remains unclear. OBJECTIVE To investigate the effect of a single high dose of vitamin D 3 on hospital length of stay in patients with COVID-19.
DESIGN, SETTING, AND PARTICIPANTS This was a multicenter, double-blind, randomized, placebo-controlled trial conducted in 2 sites in Sao Paulo, Brazil. The study included 240 hospitalized patients with COVID-19 who were moderately to severely ill at the time of enrollment from June 2, 2020, to August 27, 2020. The final follow-up was on October 7, 2020. INTERVENTIONS Patients were randomly assigned to receive a single oral dose of 200 000 IU of vitamin D 3 (n = 120) or placebo (n = 120).
MAIN OUTCOMES AND MEASURES The primary outcome was length of stay, defined as the time from the date of randomization to hospital discharge. Prespecified secondary outcomes included mortality during hospitalization; the number of patients admitted to the intensive care unit; the number of patients who required mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D, total calcium, creatinine, and C-reactive protein.
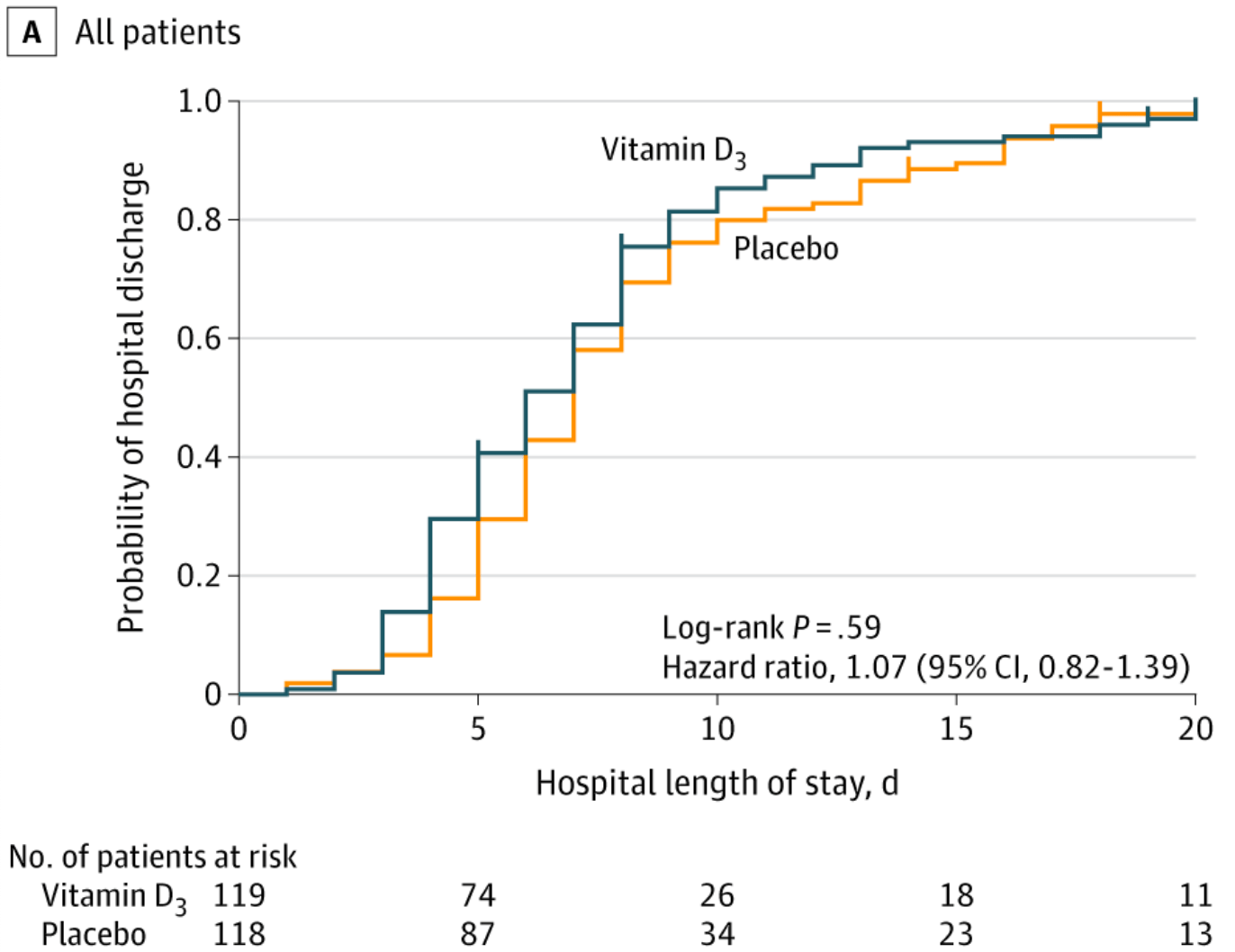
RESULTS Of 240 randomized patients, 237 were included in the primary analysis (mean [SD] age, 56.2 [14.4] years; 104 [43.9%] women; mean [SD] baseline 25-hydroxyvitamin D level, 20.9 [9.2] ng/mL). Median (interquartile range) length of stay was not significantly different between the vitamin D 3 (7.0 [4.0-10.0] days) and placebo groups (7.0 [5.0-13.0] days) (log-rank P = .59; unadjusted hazard ratio for hospital discharge, 1.07 [95% CI, 0.82-1.39]; P = .62). The difference between the vitamin D 3 group and the placebo group was not significant for in-hospital mortality (7.6% vs 5.1%; difference, 2.5% [95% CI, -4.1% to 9.2%]; P = .43), admission to the intensive care unit (16.0% vs 21.2%; difference, -5.2% [95% CI, -15.1% to 4.7%]; P = .30), or need for mechanical ventilation (7.6% vs 14.4%; difference, -6.8% [95% CI, -15.1% to 1.2%]; P = .09). Mean serum levels of 25-hydroxyvitamin D significantly increased after a single dose of vitamin D 3 vs placebo (44.4 ng/mL vs 19.8 ng/mL; difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001). There were no adverse events, but an episode of vomiting was associated with the intervention. CONCLUSIONS AND RELEVANCE Among hospitalized patients with COVID-19, a single high dose of vitamin D 3 , compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of a high dose of vitamin D 3 for treatment of moderate to severe COVID-19.
Author Contributions: Dr Pereira had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Murai
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Data Sharing Statement: See Supplement 3.
Additional Contributions: The authors are thankful to Monica Pinheiro, MD, MSc, and Roberta Costa, MSc (Ibirapuera field hospital), for assistance with the study; Cleuber Esteves Chaves, BSc (pharmacy unit of the clinical hospital), for the vitamin D 3 and placebo solution preparation; Rogério Ruscitto do Prado, PhD (Albert Einstein Hospital), for conducting statistical analyses; Cibele Russo, PhD (University of Sao Paulo), for statistical review; Mayara Diniz Santos, MS (School of Medicine of University of Sao Paulo), for technical support; all of the staff members from both centers; and all of the patients who participated in this study. None of these individuals received compensation for their participation.
References
Aibana, Huang, Aboud, Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis, PLoS Med,
doi:10.1371/journal.pmed.1002907Bacchetti, Mcculloch, Segal, Franco, Freitas et al., Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: a systematic review and meta-analysis, Biometrics,
doi:10.1097/MD.0000000000007024Bilezikian, Bikle, Hewison, Mechanisms in endocrinology: vitamin D and COVID-19, Eur J Endocrinol,
doi:10.1530/EJE-20-0665Campbell, Spector, Etten, Mathieu, Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1, J Steroid Biochem Mol Biol,
doi:10.1016/j.jsbmb.2005.06.002Carpagnano, Lecce, Quaranta, Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study, J Endocrinol Invest. Published online,
doi:10.3390/nu12113377Hernández, Nan, Fernandez-Ayala, Vitamin D status in hospitalized patients with SARS-CoV-2 infection, J Clin Endocrinol Metab,
doi:10.1210/clinem/dgaa733Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin Exp Res,
doi:10.1007/s40520-020-01570-8Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS One,
doi:10.1371/journal.pone.0239252Kearns, Alvarez, Tangpricha, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract,
doi:10.4158/EP13265.RALiu, Stenger, Li, Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, Science,
doi:10.1126/science.1123933Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ,
doi:10.1136/bmj.i6583Sabetta, Depetrillo, Cipriani, Smardin, Burns et al., Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults, PLoS One,
doi:10.1371/journal.pone.0011088DOI record:
{
"DOI": "10.1001/jama.2020.26848",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2020.26848",
"author": [
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Murai",
"given": "Igor H.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Fernandes",
"given": "Alan L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Sales",
"given": "Lucas P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Applied Physiology & Nutrition Research Group, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Pinto",
"given": "Ana J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Applied Physiology & Nutrition Research Group, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Goessler",
"given": "Karla F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Duran",
"given": "Camila S. C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Silva",
"given": "Carla B. R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Franco",
"given": "André S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Macedo",
"given": "Marina B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Dalmolin",
"given": "Henrique H. H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Baggio",
"given": "Janaina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Balbi",
"given": "Guilherme G. M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Reis",
"given": "Bruna Z.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pathology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Antonangelo",
"given": "Leila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Caparbo",
"given": "Valeria F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Applied Physiology & Nutrition Research Group, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
},
{
"name": "Food Research Center, Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Gualano",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatology Division, Hospital das Clinicas HCFMUSP, Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil"
}
],
"family": "Pereira",
"given": "Rosa M. R.",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
20
]
],
"date-time": "2021-02-20T15:58:59Z",
"timestamp": 1613836739000
},
"deposited": {
"date-parts": [
[
2021,
3,
16
]
],
"date-time": "2021-03-16T17:59:00Z",
"timestamp": 1615917540000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:01:53Z",
"timestamp": 1712070113879
},
"is-referenced-by-count": 354,
"issue": "11",
"issued": {
"date-parts": [
[
2021,
3,
16
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2021,
3,
16
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2776738/jama_murai_2021_oi_200145_1615225415.22388.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1053",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
3,
16
]
]
},
"published-print": {
"date-parts": [
[
2021,
3,
16
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1126/science.1123933",
"article-title": "Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response.",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "1770",
"issue": "5768",
"journal-title": "Science",
"key": "joi200145r1",
"volume": "311",
"year": "2006"
},
{
"DOI": "10.1001/jama.2017.8708",
"article-title": "Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children.",
"author": "Aglipay",
"doi-asserted-by": "publisher",
"first-page": "245",
"issue": "3",
"journal-title": "JAMA",
"key": "joi200145r2",
"volume": "318",
"year": "2017"
},
{
"DOI": "10.4161/auto.21154",
"article-title": "Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1.",
"author": "Campbell",
"doi-asserted-by": "publisher",
"first-page": "1523",
"issue": "10",
"journal-title": "Autophagy",
"key": "joi200145r3",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.1016/j.jsbmb.2005.06.002",
"article-title": "Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts.",
"author": "van Etten",
"doi-asserted-by": "publisher",
"first-page": "93",
"issue": "1-2",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "joi200145r4",
"volume": "97",
"year": "2005"
},
{
"DOI": "10.1016/j.gene.2018.08.017",
"article-title": "Vitamin D receptor polymorphisms and risk of enveloped virus infection: a meta-analysis.",
"author": "Laplana",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Gene",
"key": "joi200145r5",
"volume": "678",
"year": "2018"
},
{
"DOI": "10.1530/EJE-20-0665",
"article-title": "Mechanisms in endocrinology: vitamin D and COVID-19.",
"author": "Bilezikian",
"doi-asserted-by": "publisher",
"first-page": "R133",
"issue": "5",
"journal-title": "Eur J Endocrinol",
"key": "joi200145r6",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(13)70165-7",
"article-title": "Vitamin D status and ill health: a systematic review.",
"author": "Autier",
"doi-asserted-by": "publisher",
"first-page": "76",
"issue": "1",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "joi200145r7",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1371/journal.pmed.1002907",
"article-title": "Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis.",
"author": "Aibana",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLoS Med",
"key": "joi200145r8",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1136/bmj.i6583",
"article-title": "Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data.",
"author": "Martineau",
"doi-asserted-by": "publisher",
"first-page": "i6583",
"journal-title": "BMJ",
"key": "joi200145r9",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0011088",
"article-title": "Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults.",
"author": "Sabetta",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "PLoS One",
"key": "joi200145r10",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/S2213-8587(20)30183-2",
"article-title": "Vitamin-D and COVID-19: do deficient risk a poorer outcome?",
"author": "Mitchell",
"doi-asserted-by": "publisher",
"first-page": "570",
"issue": "7",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "joi200145r11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"article-title": "Vitamin D for COVID-19: a case to answer?",
"author": "Martineau",
"doi-asserted-by": "publisher",
"first-page": "735",
"issue": "9",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "joi200145r12",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"article-title": "Association of vitamin D status and other clinical characteristics with COVID-19 test results.",
"author": "Meltzer",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "JAMA Netw Open",
"key": "joi200145r13",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0239252",
"article-title": "SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels.",
"author": "Kaufman",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLoS One",
"key": "joi200145r14",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"article-title": "The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality.",
"author": "Ilie",
"doi-asserted-by": "publisher",
"first-page": "1195",
"issue": "7",
"journal-title": "Aging Clin Exp Res",
"key": "joi200145r15",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.4158/EP13265.RA",
"article-title": "Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review.",
"author": "Kearns",
"doi-asserted-by": "publisher",
"first-page": "341",
"issue": "4",
"journal-title": "Endocr Pract",
"key": "joi200145r16",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1186/1741-7015-8-17",
"article-title": "Current sample size conventions: flaws, harms, and alternatives.",
"author": "Bacchetti",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "BMC Med",
"key": "joi200145r17",
"volume": "8",
"year": "2010"
},
{
"DOI": "10.1111/j.1541-0420.2008.01004_1.x",
"article-title": "Simple, defensible sample sizes based on cost efficiency.",
"author": "Bacchetti",
"doi-asserted-by": "publisher",
"first-page": "577",
"issue": "2",
"journal-title": "Biometrics",
"key": "joi200145r18",
"volume": "64",
"year": "2008"
},
{
"DOI": "10.1097/MD.0000000000007024",
"article-title": "Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: a systematic review and meta-analysis.",
"author": "Franco",
"doi-asserted-by": "crossref",
"issue": "23",
"journal-title": "Medicine (Baltimore)",
"key": "joi200145r19",
"volume": "96",
"year": "2017"
},
{
"DOI": "10.1017/S0950268806007175",
"article-title": "Epidemic influenza and vitamin D.",
"author": "Cannell",
"doi-asserted-by": "publisher",
"first-page": "1129",
"issue": "6",
"journal-title": "Epidemiol Infect",
"key": "joi200145r20",
"volume": "134",
"year": "2006"
},
{
"article-title": "Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19.",
"author": "Carpagnano",
"journal-title": "J Endocrinol Invest",
"key": "joi200145r21",
"year": "2020"
},
{
"DOI": "10.3390/nu12113377",
"article-title": "Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study.",
"author": "Annweiler",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "Nutrients",
"key": "joi200145r22",
"volume": "12",
"year": "2020"
},
{
"article-title": "Vitamin D status in hospitalized patients with SARS-CoV-2 infection.",
"author": "Hernández",
"journal-title": "J Clin Endocrinol Metab",
"key": "joi200145r23",
"year": "2020"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2776738"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of a Single High Dose of Vitamin D<sub>3</sub> on Hospital Length of Stay in Patients With Moderate to Severe COVID-19",
"type": "journal-article",
"volume": "325"
}