Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study
Jessica J Liu, Chaim M Bell, John J Matelski, Allan S Detsky, Peter Cram
BMJ, doi:10.1136/bmj.j4619
factor for their specialty) US medical journals from 26 specialties and US Open Payments database, 2014. PARTICIPANTS 713 editors at the associate level and above identified from each journal's online masthead.
MAIN OUTCOME MEASURES All general payments (eg, personal income) and research related payments from pharmaceutical and medical device manufacturers to eligible physicians in 2014. Percentages of editors receiving payments and the magnitude of such payments were compared across journals and by specialty. Journal websites were also reviewed to determine if conflict of interest policies for editors were readily accessible.
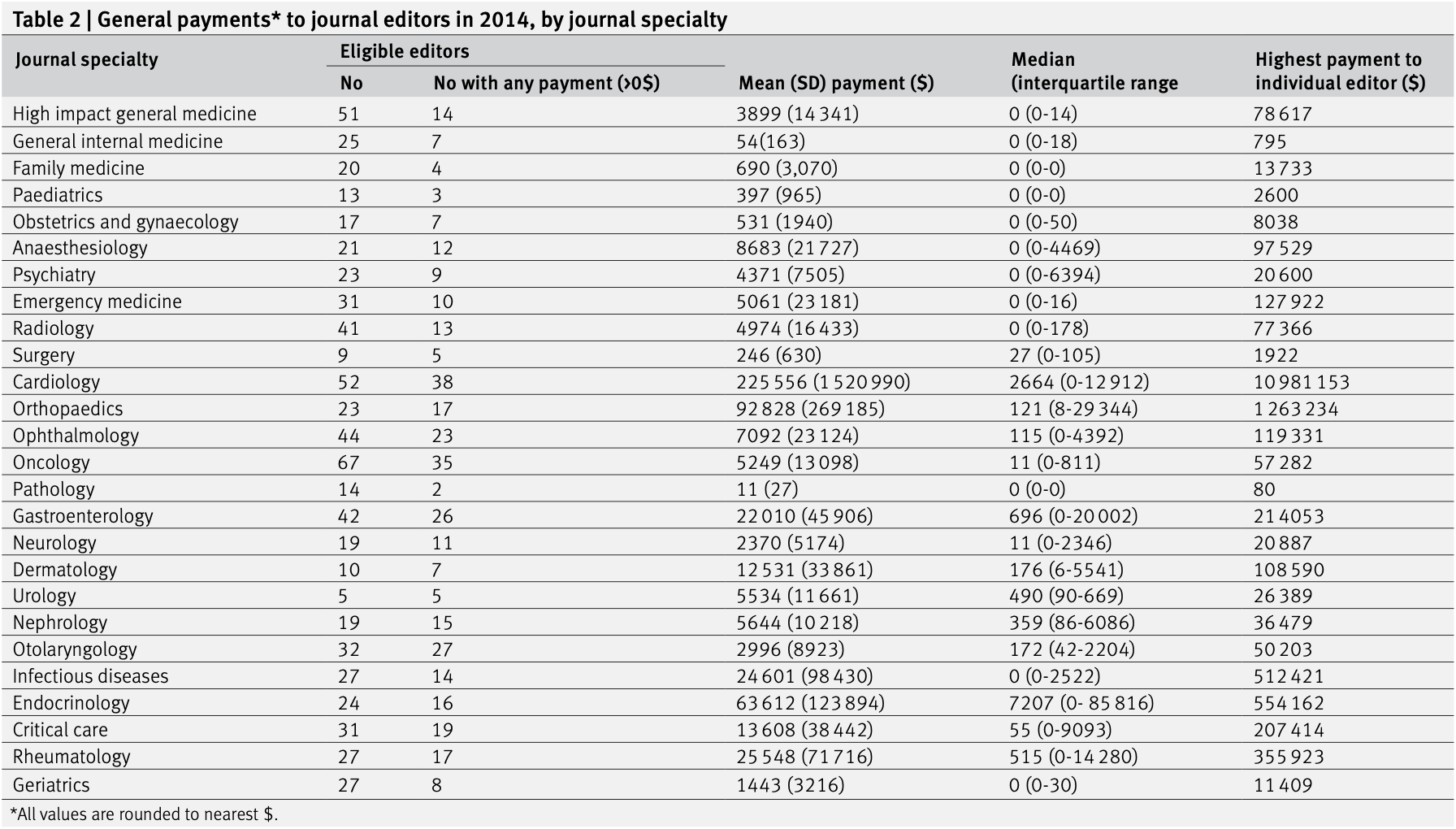
RESULTS Of 713 eligible editors, 361 (50.6%) received some (>$0) general payments in 2014, and 139 (19.5%) received research payments. The median general payment was $11 (£8; €9) (interquartile range $0-2923) and the median research payment was $0 ($0-0). The mean general payment was $28 136 (SD $415 045), and the mean research payment was $37 963 (SD $175 239). The highest median general payments were received by journal editors from endocrinology ($7207, $0-85 816), cardiology ($2664, $0-12 912), gastroenterology ($696, $0-20 002), rheumatology ($515, $0-14 280), and urology ($480, $90-669). For high impact general medicine journals, median payments were $0 ($0-14). A review of the 52 journal websites revealed that editor conflict of interest policies were readily accessible (ie, within five minutes) for 17/52 (32.7%) of journals.
CONCLUSIONS Industry payments to journal editors are common and often large, particularly for certain subspecialties.
References
Anderson, Good, Gellad, Prevalence and compensation of academic leaders, professors, and trustees on publicly traded US healthcare company boards of directors: cross sectional study, BMJ,
doi:10.1136/bmj.h4826Andreatos, Zacharioudakis, Zervou, Muhammed, Mylonakis, Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry, Medicine,
doi:10.1097/MD.0000000000005711Benson, Eyes wide open: reader and author responsibility in understanding the limits of peer review, Ann R Coll Surg Engl,
doi:10.1308/rcsann.2015.0032Blum, Freeman, Dart, Cooper, Requirements and definitions in conflict of interest policies of medical journals, JAMA,
doi:10.1001/jama.2009.1669Bosch, Pericas, Hernández, Doti, Financial, nonfinancial and editors' conflicts of interest in high-impact biomedical journals, Eur J Clin Invest,
doi:10.1111/eci.12090Davis, Müllner, Editorial independence at medical journals owned by professional associations: a survey of editors, Sci Eng Ethics,
doi:10.1007/s11948-002-0004-7Donovan, Kaplan, Navigating conflicts of interest for the medical device entrepreneur, Prog Cardiovasc Dis
Drazen, De Leeuw, Laine, Toward more uniform conflict disclosures--the updated ICMJE conflict of interest reporting form, N Engl J Med,
doi:10.1056/NEJMe1006030Fleischman, Agrawal, King, Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study, BMJ,
doi:10.1136/bmj.i4189Fontanarosa, Flanagin, Deangelis, Implementation of the ICMJE form for reporting potential conflicts of interest, JAMA,
doi:10.1001/jama.2010.1429Haivas, Schroter, Waechter, Smith, Editors' declaration of their own conflicts of interest, CMAJ,
doi:10.1503/cmaj.1031982Hannon, Chalmers, Carpiniello, Cvetanovich, Cole et al., Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the Open Payments Database[Review], J Bone Joint Surg Am,
doi:10.2106/JBJS.15.01119Iyer, Derman, Sandhu, Orthopaedics and the Physician Payments Sunshine Act: An Examination of Payments to U.S. Orthopaedic Surgeons in the Open Payments Database, J Bone Joint Surg Am
Janssen, Bredenoord, Dhert, De Kleuver, Oner et al., Potential conflicts of interest of editorial board members from five leading spine journals, PLoS One
Kirschner, Sulmasy, Kesselheim, Health policy basics: the Physician Payment Sunshine Act and the Open Payments program, Ann Intern Med,
doi:10.7326/M14-1303Krumholz, Egilman, Ross, Study of neurontin: titrate to effect, profile of safety (STEPS) trial: a narrative account of a gabapentin seeding trial, Arch Intern Med,
doi:10.1001/archinternmed.2011.241Luty, Arokiadass, Easow, Anapreddy, Preferential publication of editorial board members in medical specialty journals, J Med Ethics,
doi:10.1136/jme.2008.026740Mani, Makarević, Juengel, I publish in I edit?--Do editorial board members of urologic journals preferentially publish their own scientific work?, PLoS One,
doi:10.1371/journal.pone.0083709Marshall, Jackson, Hattangadi-Gluth, Disclosure of Industry Payments to Physicians: An Epidemiologic Analysis of Early Data From the Open Payments Program, Mayo Clin Proc,
doi:10.1016/j.mayocp.2015.10.016Mehlman, Okike, Bhandari, Kocher, Potential financial conflict of interest among physician editorial board members of orthopaedic surgery, J Bone Joint Surg Am,
doi:10.2106/JBJS.16.00227Neuman, Korenstein, Ross, Keyhani, Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study, BMJ,
doi:10.1136/bmj.d5621Norris, Holmer, Ogden, Burda, Fu, Conflicts of interest among authors of clinical practice guidelines for glycemic control in type 2 diabetes mellitus, PLoS One,
doi:10.1371/journal.pone.0075284Okike, Kocher, Wei, Mehlman, Bhandari, Accuracy of conflict-of-interest disclosures reported by physicians, N Engl J Med,
doi:10.1056/NEJMsa0807160Overgaard, Van Den Broek, Kim, Detsky, Biotechnology stock prices before public announcements: evidence of insider trading?, J Investig Med
Parreco, Donath, Kozol, Faber, Comparing industry compensation of cardiothoracic surgeons and interventional cardiologists, J Surg Res
Pisano, Golden, Schweitzer, Conflict of interest policies for academic health system leaders who work with outside corporations, JAMA,
doi:10.1001/jama.2014.788Roseman, Milette, Bero, Reporting of conflicts of interest in meta-analyses of trials of pharmacological treatments, JAMA,
doi:10.1001/jama.2011.257Ross, Hill, Egilman, Krumholz, Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation, JAMA,
doi:10.1001/jama.299.15.1800Rothenstein, Tomlinson, Tannock, Detsky, Company stock prices before and after public announcements related to oncology drugs, J Natl Cancer Inst,
doi:10.1093/jnci/djr338Samuel, Webb, Lukasiewicz, Orthopaedic Surgeons Receive the Most Industry Payments to Physicians but Large Disparities are Seen in Sunshine Act Data, Clin Orthop Relat Res,
doi:10.1007/s11999-015-4413-8Smith, Potvin, Jones, Accessibility and transparency of editor conflicts of interest policy instruments in medical journals, J Med Ethics,
doi:10.1136/medethics-2012-100524Thomas, Interventional cardiology and the medical devices industry: is there a conflict of interest?, Heart,
doi:10.1136/hrt.2007.123133Thompson, Volpe, Bridgewater, Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting, Am J Obstet Gynecol,
doi:10.1016/j.ajog.2016.06.015Tringale, Marshall, Mackey, Connor, Murphy et al., Types and Distribution of Payments From Industry to Physicians in 2015, JAMA,
doi:10.1001/jama.2017.3091{ 'indexed': {'date-parts': [[2024, 8, 22]], 'date-time': '2024-08-22T20:51:52Z', 'timestamp': 1724359912433},
'reference-count': 0,
'publisher': 'BMJ',
'license': [ { 'start': { 'date-parts': [[2017, 10, 26]],
'date-time': '2017-10-26T00:00:00Z',
'timestamp': 1508976000000},
'content-version': 'tdm',
'delay-in-days': 0,
'URL': 'http://www.bmj.org/licenses/tdm/1.0/terms-and-conditions.html'}],
'content-domain': {'domain': ['bmj.com'], 'crossmark-restriction': True},
'DOI': '10.1136/bmj.j4619',
'type': 'journal-article',
'created': { 'date-parts': [[2017, 10, 26]],
'date-time': '2017-10-26T14:15:27Z',
'timestamp': 1509027327000},
'page': 'j4619',
'update-policy': 'http://dx.doi.org/10.1136/crossmarkpolicy',
'source': 'Crossref',
'is-referenced-by-count': 73,
'title': 'Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: '
'retrospective observational study',
'prefix': '10.1136',
'author': [ {'given': 'Jessica J', 'family': 'Liu', 'sequence': 'first', 'affiliation': []},
{'given': 'Chaim M', 'family': 'Bell', 'sequence': 'additional', 'affiliation': []},
{'given': 'John J', 'family': 'Matelski', 'sequence': 'additional', 'affiliation': []},
{'given': 'Allan S', 'family': 'Detsky', 'sequence': 'additional', 'affiliation': []},
{'given': 'Peter', 'family': 'Cram', 'sequence': 'additional', 'affiliation': []}],
'member': '239',
'published-online': {'date-parts': [[2017, 10, 26]]},
'container-title': 'BMJ',
'original-title': [],
'language': 'en',
'link': [ { 'URL': 'http://data.bmj.org/tdm/10.1136/bmj.j4619',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://syndication.highwire.org/content/doi/10.1136/bmj.j4619',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2020, 9, 26]],
'date-time': '2020-09-26T01:28:29Z',
'timestamp': 1601083709000},
'score': 1,
'resource': {'primary': {'URL': 'https://www.bmj.com/lookup/doi/10.1136/bmj.j4619'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2017, 10, 26]]},
'references-count': 0,
'alternative-id': ['10.1136/bmj.j4619'],
'URL': 'http://dx.doi.org/10.1136/bmj.j4619',
'relation': {},
'ISSN': ['0959-8138', '1756-1833'],
'subject': [],
'container-title-short': 'BMJ',
'published': {'date-parts': [[2017, 10, 26]]}}