Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients
Angélica Jayk Bernal, Monica M Gomes Da Silva, Dany B Musungaie, Evgeniy Kovalchuk, Antonio Gonzalez, Virginia Delos Reyes, Alejandro Martín-Quirós, Yoseph Caraco, Angela Williams-Diaz, Michelle L Brown, Jiejun Du, Alison Pedley, Christopher Assaid, Julie Strizki, Jay A Grobler, Hala H Shamsuddin, Robert Tipping, Hong Wan, Amanda Paschke, Joan R Butterton, Matthew G Johnson, Carisa De Anda
New England Journal of Medicine, doi:10.1056/nejmoa2116044
BACKGROUND New treatments are needed to reduce the risk of progression of coronavirus disease 2019 (Covid-19). Molnupiravir is an oral, small-molecule antiviral prodrug that is active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
METHODS We conducted a phase 3, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of treatment with molnupiravir started within 5 days after the onset of signs or symptoms in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed Covid-19 and at least one risk factor for severe Covid-19 illness. Participants in the trial were randomly assigned to receive 800 mg of molnupiravir or placebo twice daily for 5 days. The primary efficacy end point was the incidence hospitalization or death at day 29; the incidence of adverse events was the primary safety end point. A planned interim analysis was performed when 50% of 1550 participants (target enrollment) had been followed through day 29.
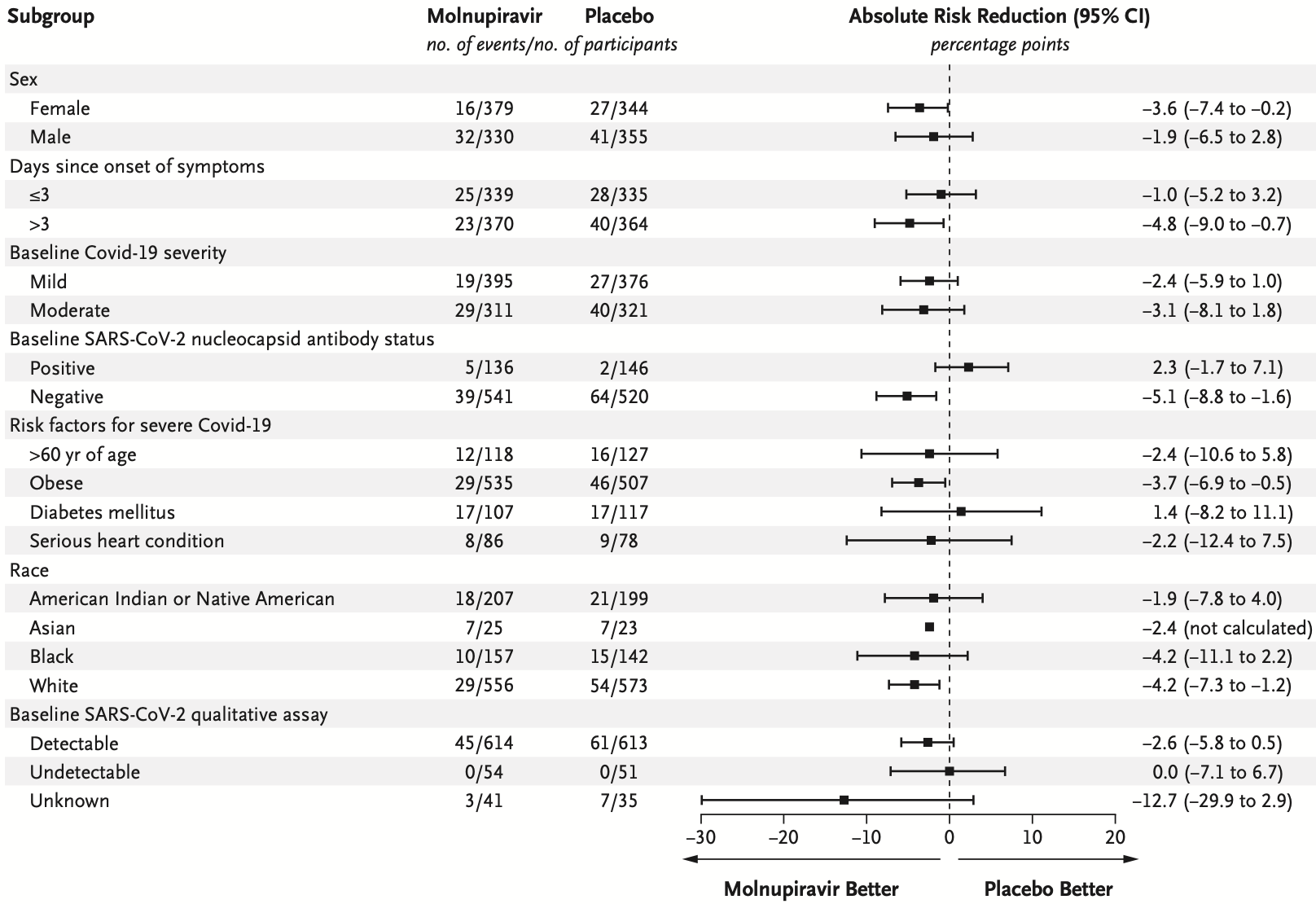
RESULTS A total of 1433 participants underwent randomization; 716 were assigned to receive molnupiravir and 717 to receive placebo. With the exception of an imbalance in sex, baseline characteristics were similar in the two groups. The superiority of molnupiravir was demonstrated at the interim analysis; the risk of hospitalization for any cause or death through day 29 was lower with molnupiravir (28 of 385 participants [7.3%]) than with placebo (53 of 377 [14.1%]) (difference, −6.8 percentage points; 95% confidence interval, −11.3 to −2.4; P = 0.001). In the analysis of all participants who had undergone randomization, the percentage of participants who were hospitalized or died through day 29 was lower in the molnupiravir group than in the placebo group (6.8% [48 of 709] vs. 9.7% [68 of 699]; difference, −3.0 percentage points; 95% confidence interval, −5.9 to −0.1). Results of subgroup analyses were largely consistent with these overall results; in some subgroups, such as patients with evidence of previous SARS-CoV-2 infection, those with low baseline viral load, and those with diabetes, the point estimate for the difference favored placebo. One death was reported in the molnupiravir group and 9 were reported in the placebo group through day 29. Adverse events were reported in 216 of 710 participants (30.4%) in the molnupiravir group and 231 of 701 (33.0%) in the placebo group.
CONCLUSIONS Early treatment with molnupiravir reduced the risk of hospitalization or death in at-risk, unvaccinated adults with Covid-19. (Funded by Merck Sharp and Dohme; MOVe-OUT ClinicalTrials.gov number, NCT04575597.
Supported by Merck Sharp and Dohme, a subsidiary of Merck, Kenilworth, NJ. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available with the full text of this article at NEJM.org. We thank the participants and their families and caregivers for their participation in this trial and Wendy Painter, M.D., and Wayne Holman, M.D., both of Ridgeback Biotherapeutics, Miami, for scientific discussions. Medical writing assistance was provided by Dominik J. Wolf, M.Sc., who wrote the first draft of the manuscript under guidance from the authors, and editorial assistance was provided by Karyn Davis, B.S., both of Merck Sharp and Dohme, a subsidiary of Merck, Kenilworth, NJ. n engl j med nejm.org
Appendix
References
Abdelnabi, Foo, Jonghe, Maes, Weynand et al., Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model, J Infect Dis
Agostini, Pruijssers, Chappell, Small-molecule antiviral beta-d-N 4 -hydroxycytidine inhibits a proofreadingintact coronavirus with a high genetic barrier to resistance, J Virol
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19, NEJM Evid,
doi:10.1056/EVIDoa2100044Bajema, Dahl, Prill, Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalization -five veterans affairs medical centers, United States, February 1, MMWR Morb Mortal Wkly Rep
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults, NEJM Evid,
doi:10.1056/EVIDoa2100043Chawla, Cao, Stone, Modelbased dose selection for the phase 3 evaluation of molnupiravir (MOV) in the treatment of COVID-19 in adults
Cohen, Wohl, Fischer, Smith, Eron, Outpatient treatment of SARS-CoV-2 infection to prevent COVID-19 progression, Clin Infect Dis
Cowman, Guo, Pirofski, Post-severe acute respiratory syndrome coronavirus 2 monoclonal antibody treatment hospitalizations as a sentinel for emergence of viral variants in New York City, Open Forum Infect Dis
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Gordon, Tchesnokov, Schinazi, Götte, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J Biol Chem
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Grobler, Strizki, Murgolo, Molnupiravir maintains antiviral activity against SARS-CoV-2 variants in vitro and in early clinical studies
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Horby, Mafham, Peto, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial,
doi:10.1101/2021.06.15.21258542v1Hurt, Wheatley, Neutralizing antibody therapeutics for COVID-19, Viruses
Hwang, Shih, Cani, Group sequential designs using a family of type I error probability spending functions, Stat Med
Kabinger, Stiller, Schmitzová, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Khoo, Fitzgerald, Fletcher, Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase I, open-label, dose-escalating, randomized controlled study, J Antimicrob Chemother
Ko, Danielson, Town, Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system, Clin Infect Dis
Kompaniyets, Goodman, Belay, Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death -United States, March-December 2020, MMWR Morb Mortal Wkly Rep
Malone, Campbell, Molnupiravir: coding for catastrophe, Nat Struct Mol Biol
Miettinen, Nurminen, Comparative analysis of two rates, Stat Med
Nguyen, Nguyen, Corlin, Allen, Chung, Changes in COVID-19 vaccination receipt and intention to vaccinate by socioeconomic characteristics and geographic area, United States, January 6, Ann Med
Painter, Holman, Bush, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broadspectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother
Pogue, Lauring, Gandhi, Monoclonal antibodies for early treatment of COVID-19 in a world of evolving SARS-CoV-2 mutations and variants, Open Forum Infect Dis
Rosenberg, Holtgrave, Dorabawila, New COVID-19 cases and hospitalizations among adults, by vaccination status -New York, May 3, MMWR Morb Mortal Wkly Rep
Sheahan, Sims, Zhou, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med
Stokes, Zambrano, Anderson, Coronavirus disease 2019 case surveillance -United States, January 22, MMWR Morb Mortal Wkly Rep
Tenforde, Kim, Lindsell, Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network -United States, March-June 2020, MMWR Morb Mortal Wkly Rep
Tenforde, Self, Naioti, Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults -United States, March-July 2021, MMWR Morb Mortal Wkly Rep
Urakova, Kuznetsova, Crossman, β-d-N 4 -hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome, J Virol
Wagner, Saad-Roy, Morris, Vaccine nationalism and the dynamics and control of SARS-CoV-2, Science
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Yoon, Toots, Lee, Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1056/nejmoa2116044",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/nejmoa2116044",
"alternative-id": [
"10.1056/NEJMoa2116044"
],
"author": [
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Jayk Bernal",
"given": "Angélica",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Gomes da Silva",
"given": "Monica M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Musungaie",
"given": "Dany B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Kovalchuk",
"given": "Evgeniy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Gonzalez",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Delos Reyes",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Martín-Quirós",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Caraco",
"given": "Yoseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Williams-Diaz",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Brown",
"given": "Michelle L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Du",
"given": "Jiejun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Pedley",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Assaid",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Strizki",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Grobler",
"given": "Jay A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Shamsuddin",
"given": "Hala H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Tipping",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Wan",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Paschke",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Butterton",
"given": "Joan R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "Johnson",
"given": "Matthew G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From IMAT Oncomédica, Monteria, Colombia (A.J.B.); the Department of Public Health, Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil (M.M.G.S.); Jongaie Research, Pretoria, South Africa (D.B.M.); Medical Research Institute, St. Petersburg, Russia (E.K.); Advanced Research for Health Improvement, Immokalee, FL (A.G.); Lung Center of the Philippines, Quezon City, Philippines (V.D.R.); Hospital Universitario La Paz, IdiPAZ, Madrid (A.M.-Q.); Clinical Pharmacology Unit, Hadassah–Hebrew..."
}
],
"family": "De Anda",
"given": "Carisa",
"sequence": "additional"
}
],
"container-title": [
"New England Journal of Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T22:00:26Z",
"timestamp": 1639692026000
},
"deposited": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T22:00:28Z",
"timestamp": 1639692028000
},
"funder": [
{
"DOI": "10.13039/100004334",
"doi-asserted-by": "publisher",
"name": "Merck"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
17
]
],
"date-time": "2021-12-17T05:57:44Z",
"timestamp": 1639720664511
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0028-4793"
},
{
"type": "electronic",
"value": "1533-4406"
}
],
"issued": {
"date-parts": [
[
2021,
12,
16
]
]
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T00:00:00Z",
"timestamp": 1639612800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2116044",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
12,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
16
]
]
},
"publisher": "Massachusetts Medical Society",
"reference-count": 31,
"references-count": 31,
"relation": {},
"score": 1,
"short-container-title": [
"N Engl J Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients"
],
"type": "journal-article"
}