Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease
Juan Manuel Figueroa, Mónica Edith Lombardo, Ariel Dogliotti, Luis Pedro Flynn, Robert Giugliano, Guido Simonelli, Ricardo Valentini, Agñel Ramos, Pablo Romano, Marcelo Marcote, Alicia Michelini, Alejandro Salvado, Emilio Sykora, Cecilia Kniz, Marcelo Kobelinsky, David Manuel Salzberg, Diana Jerusalinsky, Osvaldo Uchitel
International Journal of General Medicine, doi:10.2147/ijgm.s328486
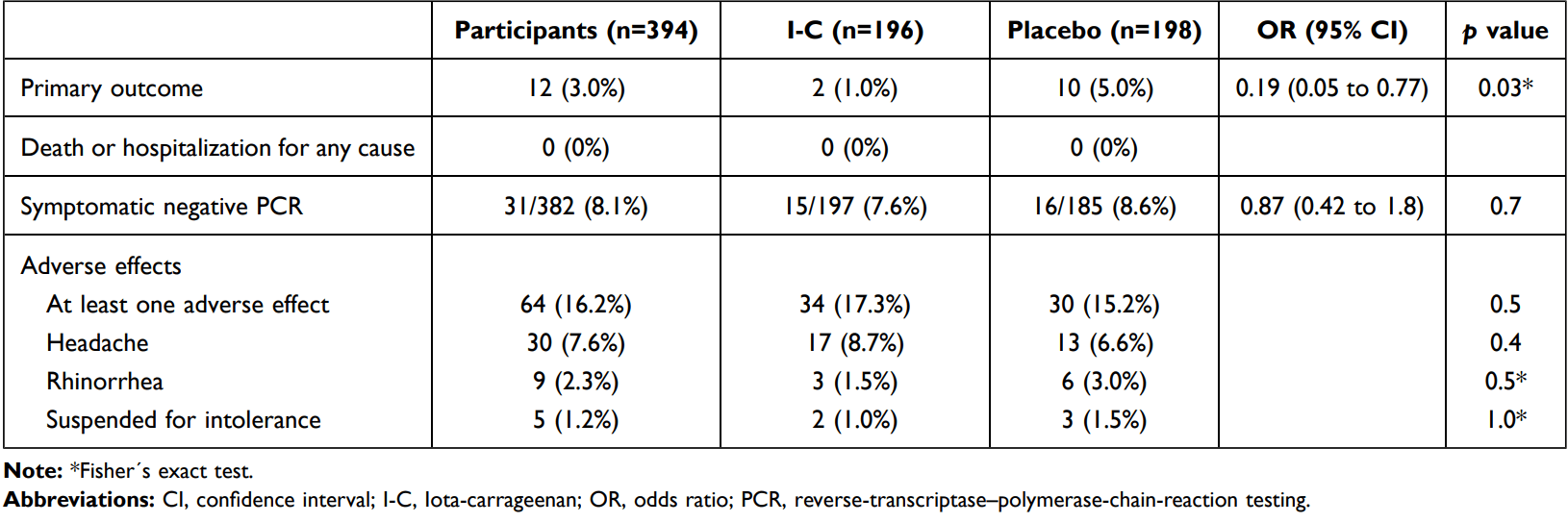
Background: Iota-Carrageenan (I-C) is a sulfate polysaccharide synthesized by red algae, with demonstrated antiviral activity and clinical efficacy as nasal spray in the treatment of common cold. In vitro, I-C inhibits SARS-CoV-2 infection in cell culture. Research Question: Can a nasal spray with Iota-Carrageenan be useful in the prophylaxis of COVID-19 in health care workers managing patients with COVID-19 disease? Study Design and Methods: This is a pilot pragmatic multicenter, randomized, doubleblind, placebo-controlled study assessing the use of a nasal spray containing I-C in the prophylaxis of COVID-19 in hospital personnel dedicated to care of COVID-19 patients. Clinically healthy physicians, nurses, kinesiologists and other health care providers managing patients hospitalized for COVID-19 were assigned in a 1:1 ratio to receive four daily doses of I-C spray or placebo for 21 days. The primary end point was clinical COVID-19, as confirmed by reverse transcriptase polymerase chain reaction testing, over a period of 21 days. The trial is registered at ClinicalTrials.gov (NCT04521322). Results: A total of 394 individuals were randomly assigned to receive I-C or placebo. Both treatment groups had similar baseline characteristics. The incidence of COVID-19 differs significantly between subjects receiving the nasal spray with I-C (2 of 196 [1.0%]) and those receiving placebo (10 of 198 [5.0%]). Relative risk reduction: 79.8% (95% CI 5.3 to 95.4; p=0.03). Absolute risk reduction: 4% (95% CI 0.6 to 7.4). Interpretation: In this pilot study a nasal spray with I-C showed significant efficacy in preventing COVID-19 in health care workers managing patients with COVID-19 disease. Clinical Trials Registration: NCT04521322.
Funding The study did not receive any support for hospitals, staff or patients involved. Publication and administrative costs were supported by: Programa de articulación y fortalecimiento federal de las capacidades en ciencia y tecnología COVID-
International Journal of General Medicine
Dovepress
References
Ahmadi, Moghadamtousi, Abubakar, Zandi, Antiviral potential of algae polysaccharides isolated from marine sources: a review, Biomed Res Int,
doi:10.1155/2015/825203Barnabas, Brown, Bershteyn, Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial, Ann Intern Med,
doi:10.7326/M20-6519Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19, N Engl J Med,
doi:10.1056/NEJMoa2016638Eccles, Meier, Jawad, Weinmüllner, Grassauer et al., Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold, Respir Res,
doi:10.1186/1465-9921-11-108Eccles, Winther, Johnston, Robinson, Trampisch et al., Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial, Respir Res,
doi:10.1186/s12931-015-0281-8Figueroa, TCPDF
Graf, Bernkop-Schnürch, Egyed, Koller, Prieschl-Grassauer et al., Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int J Gen Med,
doi:10.2147/IJGM.S167123Grassauer, Weinmuellner, Meier, Pretsch, Prieschl-Grassauer et al., Iota-Carrageenan is a potent inhibitor of rhinovirus infection, Virol J,
doi:10.1186/1743-422X-5-107Griffin, Brennan-Rieder, Ngo, The importance of understanding the stages of COVID-19 in treatment and trials, AIDS Rev,
doi:10.24875/AIDSRev.200001261Hemilä, Chalker, Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: re-analysis of randomized trial data, Pharmacol Res Perspect,
doi:10.1002/prp2.810Klimyte, Smith, Oreste, Lembo, Dutch, Inhibition of human metapneumovirus binding to heparan sulfate blocks infection in human lung cells and airway tissues, J Virol,
doi:10.1128/JVI.01362-16Lauer, Grantz, Bi, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med,
doi:10.7326/M20-0504Logunov, Dolzhikova, Shcheblyakov, -Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled Phase 3 trial in Russia, Lancet,
doi:10.1016/S0140-6736(21)00234-8Ludwig, Enzenhofer, Schneider, Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial, Respir Res,
doi:10.1186/1465-9921-14-124Mitjà, Corbacho-Monné, Ubals, BCN-PEP-CoV2 research group. A cluster-randomized trial of hydroxychloroquine for prevention of covid-19, N Engl J Med,
doi:10.1056/NEJMoa2021801Morokutti-Kurz, Graf, Grassauer, Prieschl-Grassauer, SARS-CoV-2 in-vitro neutralization assay reveals inhibition of virus entry by iota-carrageenan, bioRxiv,
doi:10.1101/2020.07.28.224733Niriella, Ediriweera, Silva, Hydroxychloroquine for post-exposure prophylaxis of COVID-19 among naval personnel in Sri Lanka: study protocol for a randomized, controlled trial, Trials,
doi:10.1186/s13063-020-04659-7Polack, Thomas, Kitchin, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med,
doi:10.1056/NEJMoa2034577Schütz, Conzelmann, Fois, Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am J Physiol Lung Cell Mol Physiol,
doi:10.1152/ajplung.00552.2020Song, Peng, Wang, Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2, Food Funct,
doi:10.1039/d0fo02017fVarese, Ceballos, Palacios, Figueroa, Dugour, Iotacarrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model, bioRxiv,
doi:10.1101/2021.04.27.441512Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Weiner, Food additive carrageenan: part II: a critical review of carrageenan in vivo safety studies, International Journal of General Medicine,
doi:10.3109/10408444.2013.861798Xin, Wong, Murphy, the incubation period distribution of coronavirus disease 2019: a systematic review and meta-analysis, Clin Infect Dis,
doi:10.1093/cid/ciab501Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med,
doi:10.1056/NEJMc2001737DOI record:
{
"DOI": "10.2147/ijgm.s328486",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S328486",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5680-1486",
"affiliation": [],
"authenticated-orcid": true,
"family": "Figueroa",
"given": "Juan Manuel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lombardo",
"given": "Mónica Edith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dogliotti",
"given": "Ariel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flynn",
"given": "Luis Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giugliano",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simonelli",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentini",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramos",
"given": "Agñel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romano",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcote",
"given": "Marcelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michelini",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvado",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sykora",
"given": "Emilio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kniz",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kobelinsky",
"given": "Marcelo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9861-4267",
"affiliation": [],
"authenticated-orcid": true,
"family": "Salzberg",
"given": "David Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jerusalinsky",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uchitel",
"given": "Osvaldo",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
30
]
],
"date-time": "2021-09-30T13:25:31Z",
"timestamp": 1633008331000
},
"deposited": {
"date-parts": [
[
2021,
9,
30
]
],
"date-time": "2021-09-30T13:25:36Z",
"timestamp": 1633008336000
},
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T18:24:49Z",
"timestamp": 1712687089512
},
"is-referenced-by-count": 37,
"issued": {
"date-parts": [
[
2021,
10
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=74193",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=74193",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "6277-6286",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
10
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMoa2034577",
"author": "Polack",
"doi-asserted-by": "publisher",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "ref1",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00234-8",
"author": "Logunov",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "Lancet",
"key": "ref2",
"volume": "397",
"year": "2021"
},
{
"key": "ref3",
"unstructured": "World Health Organization Draft landscape of COVID-19 candidate vaccines; Jan 22, 2021. Available from: https://www.who.int/publications/m/item/draft-landscape-of-cOVID-19-candidate-vaccines. Accessed September 16, 2021."
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"author": "Voysey",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Lancet",
"key": "ref4",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035389",
"author": "Baden",
"doi-asserted-by": "publisher",
"first-page": "403",
"journal-title": "N Engl J Med",
"key": "ref5",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1080/21645515.2016.1165908",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "2064",
"journal-title": "Hum Vaccin Immunother",
"key": "ref6",
"volume": "12",
"year": "2016"
},
{
"DOI": "10.1155/2015/825203",
"author": "Ahmadi",
"doi-asserted-by": "publisher",
"first-page": "825203",
"journal-title": "Biomed Res Int",
"key": "ref7",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0014320",
"author": "Leibbrandt",
"doi-asserted-by": "publisher",
"first-page": "e14320",
"journal-title": "PLoS One",
"key": "ref8",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2011.08.010",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Antiviral Res",
"key": "ref9",
"volume": "92",
"year": "2011"
},
{
"DOI": "10.1186/1743-422X-5-107",
"author": "Grassauer",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "Virol J",
"key": "ref10",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1016/j.virol.2007.01.043",
"author": "Grassauer",
"doi-asserted-by": "publisher",
"first-page": "473",
"journal-title": "Virology",
"key": "ref11",
"volume": "363",
"year": "2007"
},
{
"DOI": "10.1371/journal.ppat.0020069",
"author": "Buck",
"doi-asserted-by": "publisher",
"first-page": "e69",
"journal-title": "PLoS Pathog",
"key": "ref12",
"volume": "2",
"year": "2006"
},
{
"DOI": "10.1128/JVI.01362-16",
"author": "Klimyte",
"doi-asserted-by": "publisher",
"first-page": "9237",
"journal-title": "J Virol",
"key": "ref13",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1002/prp2.810",
"author": "Hemilä",
"doi-asserted-by": "publisher",
"first-page": "e00810",
"journal-title": "Pharmacol Res Perspect",
"key": "ref14",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/1465-9921-11-108",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "Respir Res",
"key": "ref15",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1186/1465-9921-14-124",
"author": "Ludwig",
"doi-asserted-by": "publisher",
"first-page": "124",
"journal-title": "Respir Res",
"key": "ref16",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"author": "Eccles",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Respir Res",
"key": "ref17",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"author": "Schütz",
"doi-asserted-by": "publisher",
"first-page": "L750",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "ref18",
"volume": "320",
"year": "2021"
},
{
"DOI": "10.1039/d0fo02017f",
"author": "Song",
"doi-asserted-by": "publisher",
"first-page": "7415",
"journal-title": "Food Funct",
"key": "ref19",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.28.224733",
"author": "Morokutti-Kurz",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref20",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.19.225854",
"author": "Bansal",
"doi-asserted-by": "publisher",
"journal-title": "BioRxiv",
"key": "ref21",
"year": "2020"
},
{
"DOI": "10.1101/2021.04.27.441512",
"author": "Varese",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref22",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2001737",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "1177",
"journal-title": "N Engl J Med",
"key": "ref23",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.2147/IJGM.S167123",
"author": "Graf",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Int J Gen Med",
"key": "ref24",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.7326/M20-0504",
"author": "Lauer",
"doi-asserted-by": "publisher",
"first-page": "577",
"journal-title": "Ann Intern Med",
"key": "ref25",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab501",
"author": "Xin",
"doi-asserted-by": "publisher",
"first-page": "ciab501",
"journal-title": "Clin Infect Dis",
"key": "ref26",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2016638",
"author": "Boulware",
"doi-asserted-by": "publisher",
"first-page": "517",
"journal-title": "N Engl J Med",
"key": "ref27",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7326/M20-6519",
"author": "Barnabas",
"doi-asserted-by": "publisher",
"first-page": "344",
"journal-title": "Ann Intern Med",
"key": "ref28",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021801",
"author": "Mitjà",
"doi-asserted-by": "publisher",
"first-page": "417",
"journal-title": "N Engl J Med",
"key": "ref29",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04659-7",
"author": "Niriella",
"doi-asserted-by": "publisher",
"first-page": "748",
"journal-title": "Trials",
"key": "ref30",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.24875/AIDSRev.200001261",
"author": "Griffin",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "AIDS Rev",
"key": "ref31",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3109/10408444.2013.861798",
"author": "Weiner",
"doi-asserted-by": "publisher",
"first-page": "244",
"journal-title": "Crit Rev Toxicol",
"key": "ref32",
"volume": "44",
"year": "2014"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.04.13.21255409",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/efficacy-of-a-nasal-spray-containing-iota-carrageenan-in-the-postexpos-peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease",
"type": "journal-article",
"volume": "Volume 14"
}