Abstract: Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19
Pandemic
Ahmed Elgazzar ( dr_ahmed_elgazzar@yahoo.com )
Banha University
Abdelaziz Eltaweel
Benha University
Shaimaa Abo Youssef
Banha University
Basma Hany
Community Medicine Department, Faculty of Medicine, Benha University, Egypt.
Mohy Hafez
Benha Faculty of medicine
Hany Moussa
Kafr Elsheikh University, Faculty of medicine, Chest department
Article
Keywords: Ivermectin, COVID-19, Hydroxychloroquine
Posted Date: December 28th, 2020
DOI: https://doi.org/10.21203/rs.3.rs-100956/v3
License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License
EDITORIAL NOTE:
Research Square withdrew this preprint on 14 July, 2021 due to an expression of concern communicated directly to our staff. These concerns are now
under formal investigation. Detailed information on the reasons for this withdrawal can be found here.
(note updated 12 March, 2022)
Page 1/10
Abstract
Background: Up-to-date, there is no recognized effective treatment or vaccine for the treatment of COVID-19 that emphasize urgency
around distinctive effective therapies. This study aims to evaluate the anti-parasitic medication efficacy "Ivermectin" plus standard care in the treatment of
mild/moderate and severely ill cases with COVID 19 infection, as well as prophylaxis of health care and/ or household contacts.
Subject and methods: 600 subjects; 400 symptomatic confirmed COVID-19 patients and 200 health care and household contacts distributed over 6 groups;
Group I: 100 patients with mild/moderate COVID-19 infection received a 4-days course of Ivermectin plus standard of care; Group II: 100 patients with
mild/moderate COVID-19 infection received hydroxychloroquine plus standard care; Group III: 100 patients with severe COVID-19 infection received Ivermectin
plus standar care; Group IV: 100 patients with Severe COVID-19 infection received hydroxychloroquine plus standard care. Routine laboratory investigations
and rT-PCR, were reported before and after initiation of treatment. Group V stick to personal protective measures (PPM ) plus Ivermectin o.4mg / kg on empty
stomach to be repeated after one week, and group VI stick to PPM only .Both groups V&VI were followed for two weeks ..
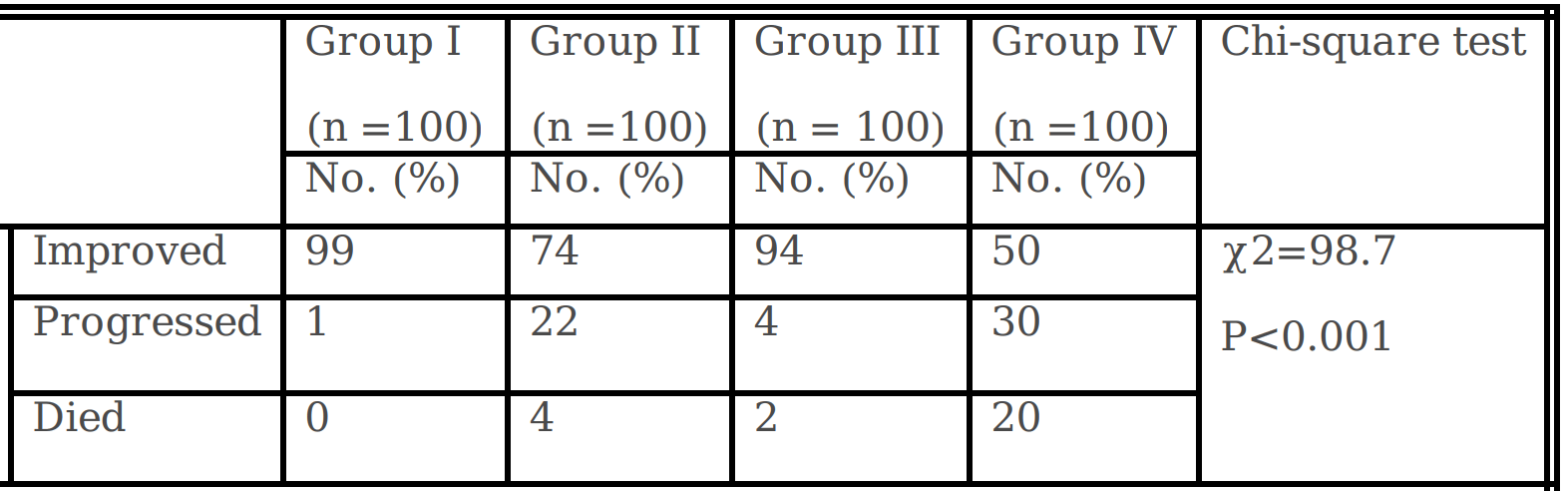
Results: Patients received ivermectin early reported substantial recovery of laboratory investigations; and significant reduction in rT-PCR conversion days. A
substantial improvement and reduction in mortality rate in Ivermectin treated groups; group I (99% & 0.0%, respectively) and group III (94% & 2.0% respectively)
versus hydroxychloroquine plus standard care treated groups; group II (74% and 4%, respectively) and group IV (50% and 20%, respectively). Ivermectin had
significantly reduced the incidence of infection in health care and household contacts up to 2% compared to 10% in non ivermectin group when used as a
prophylaxis.
Conclusion: Early addition of Ivermectin to standard care is very effective drug for treatment of COVID-19 patients with significant reduction in mortality,rt-PCR
conversion days , recovery time hospital stay compared to Hydroxychloroquine plus standard care. Early use of Ivermectin is very useful for controlling COVID
19 infections; prophylaxis and improving cytokines storm
DOI record:
{
"DOI": "10.21203/rs.3.rs-100956/v2",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-100956/v2",
"abstract": "<title>Abstract</title>\n <p><bold>Background:</bold> Up-to-date, there is no recognized effective treatment or vaccine for the treatment of COVID-19 that emphasize urgency around distinctive effective therapies. This study aims to evaluate the anti-parasitic medication efficacy \"Ivermectin\" plus standard care ((Azithromycin, vitamin C, Zinc, Lactoferrin & Acetylcystein & prophylactic or therapeutic anticoagulation if D-dimer > 1000)) in the treatment of mild/moderate and severely ill cases with COVID 19 infection, as well as prophylaxis of health care and/ or household contacts in comparison to the <italic>Hydroxychloroquine</italic> plus standard treatment.<bold>Subject and methods:</bold> 600 subjects; 400 symptomatic confirmed COVID-19 patients and 200 health care and household contacts distributed over 6 groups; <italic>Group I</italic><bold>:</bold> 100 patients with mild/moderate COVID-19 infection received a 4-days course of Ivermectin plus standard of care; <italic>Group II:</italic> 100 patients with mild/moderate COVID-19 infection received hydroxychloroquine plus standard of care; <italic>Group III:</italic> 100 patients with severe COVID-19 infection received Ivermectin plus standard of care; <italic>Group IV</italic>: 100 patients with Severe COVID-19 infection received hydroxychloroquine plus standard of care. Routine laboratory investigations and RT-PCR, were reported before and after initiation of treatment. <italic>Group V stick to PPE plus Ivermectin 400mcg / kg on empty stomach to be repeated after one week, and group VI stick to PPE only (personal protective equipment) .Both groups V&VI were followed for two weeks ..</italic><bold>Results:</bold> Patients received ivermectin reported substantial recovery of laboratory investigations; and significant reduction in RT-PCR conversion days. A substantial improvement and reduction in mortality rate in Ivermectin treated groups; group I (mild/moderate cases), (99%, and 0.0%, respectively) and group III (severe cases), (94%, and 2.0% respectively) versus hydroxychloroquine plus standard care treated groups; group II (mild/moderate cases), (74% and 4%, respectively) and group IV (severe cases) (50% and 20%, respectively). Ivermectin had significantly reduced the incidence of infection in health care and household contacts up to 2% compared to 10% in non ivermectin group when used as a prophylaxis.<bold>Conclusion:</bold><italic> </italic>Addition of Ivermectin to standard care is very effective drug for treatment of COVID-19 patients with significant reduction in mortality compared to Hydroxychloroquine plus standard treatment only. Early use of Ivermectin is very useful for controlling COVID 19 infections; prophylaxis and improving cytokines storm</p>",
"accepted": {
"date-parts": [
[
2020,
10,
30
]
]
},
"author": [
{
"affiliation": [
{
"name": "Banha University"
}
],
"family": "Elgazzar",
"given": "Ahmed",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Community Medicine Department, Faculty of Medicine, Benha University, Egypt."
}
],
"family": "Hany",
"given": "Basma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Banha University"
}
],
"family": "Youssef",
"given": "Shaimaa Abo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Benha Faculty of medicine"
}
],
"family": "Hafez",
"given": "Mohy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kafr Elsheikh University, Faculty of medicine, Chest department"
}
],
"family": "Moussa",
"given": "Hany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Benha University"
}
],
"family": "eltaweel",
"given": "Abdelaziz",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
11,
16
]
],
"date-time": "2020-11-16T23:00:32Z",
"timestamp": 1605567632000
},
"deposited": {
"date-parts": [
[
2024,
5,
10
]
],
"date-time": "2024-05-10T00:57:45Z",
"timestamp": 1715302665000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
5,
10
]
],
"date-time": "2024-05-10T01:10:18Z",
"timestamp": 1715303418873
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 16,
"issued": {
"date-parts": [
[
2020,
11,
16
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
16
]
],
"date-time": "2020-11-16T00:00:00Z",
"timestamp": 1605484800000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-100956/v2",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-100956/v2.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
11,
16
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2020,
11,
16
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {
"is-version-of": [
{
"asserted-by": "subject",
"id": "10.21203/rs.3.rs-100956/v1",
"id-type": "doi"
},
{
"asserted-by": "subject",
"id": "10.21203/rs.3.rs-100956/v3",
"id-type": "doi"
},
{
"asserted-by": "subject",
"id": "10.21203/rs.3.rs-100956/v4",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-100956/v2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "WITHDRAWN: Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic",
"type": "posted-content"
}