Is COVID-19 Infection a Multiorganic Disease? Focus on Extrapulmonary Involvement of SARS-CoV-2
Gauthier Duloquin, Thibaut Pommier, Marjolaine Georges, Maurice Giroud, Charles Guenancia, Yannick Béjot, Gabriel Laurent, Claudio Rabec
Journal of Clinical Medicine, doi:10.3390/jcm13051397
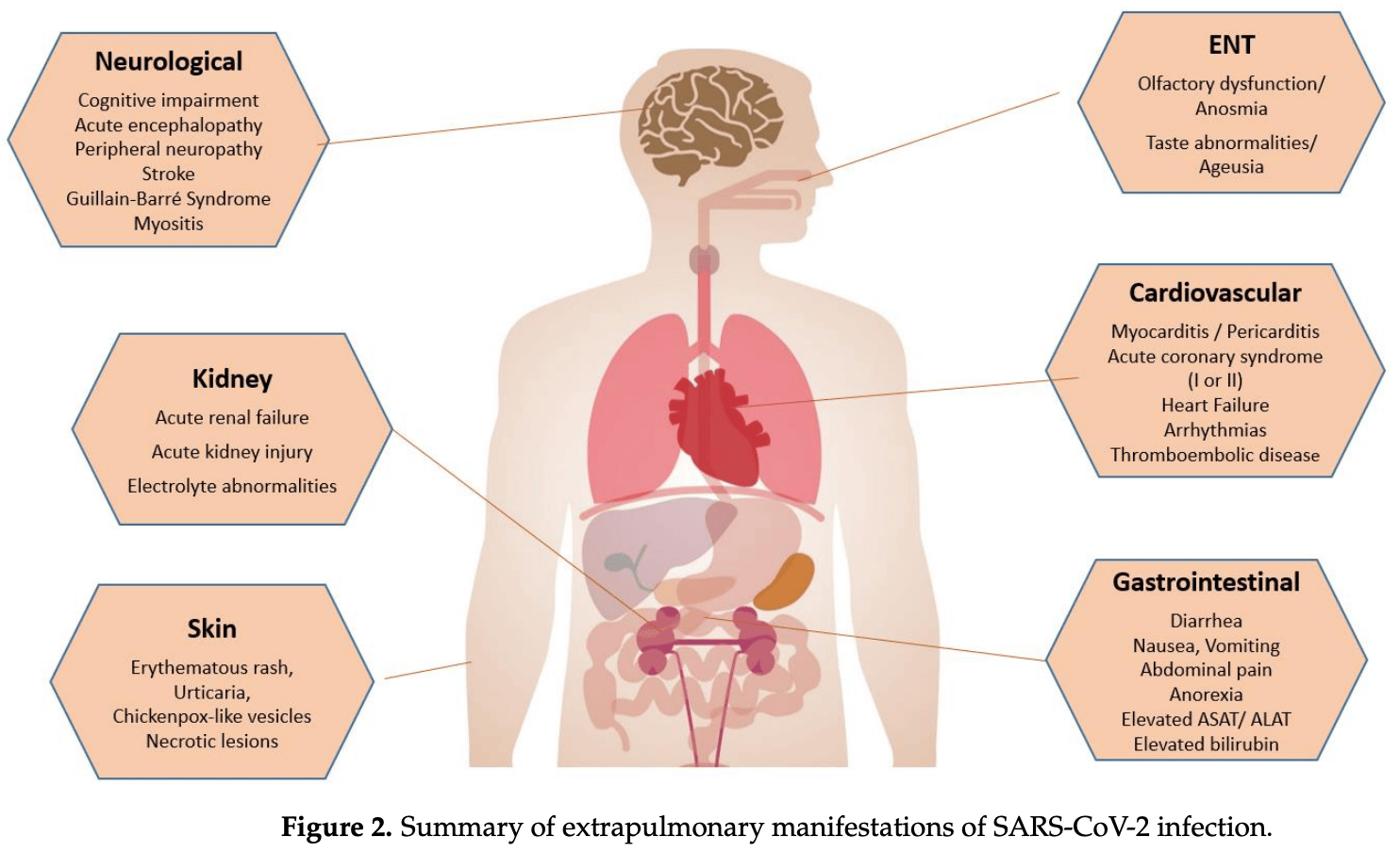
First described in December 2019 in Wuhan (China), COVID-19 disease rapidly spread worldwide, constituting the biggest pandemic in the last 100 years. Even if SARS-CoV-2, the agent responsible for COVID-19, is mainly associated with pulmonary injury, evidence is growing that this virus can affect many organs, including the heart and vascular endothelial cells, and cause haemostasis, CNS, and kidney and gastrointestinal tract abnormalities that can impact in the disease course and prognosis. In fact, COVID-19 may affect almost all the organs. Hence, SARS-CoV-2 is essentially a systemic infection that can present a large number of clinical manifestations, and it is variable in distribution and severity, which means it is potentially life-threatening. The goal of this comprehensive review paper in the series is to give an overview of non-pulmonary involvement in COVID-19, with a special focus on underlying pathophysiological mechanisms and clinical presentation.
Conflicts of Interest: Yannick Béjot received honoraria for lectures or consulting fees from BMS, Pfizer, Medtronic, Amgen, Servier, Novo Nordisk, and Novartis, outside the submitted work. Claudio Rabec received honoraria for lectures or consulting fees from Vitalaire, Resmed, Philips, Asten Santé, Lowenstein Medical, outside the submitted work. Other authors declare no competing interests.
References
Aghagoli, Gallo Marin, Soliman, Sellke, Cardiac involvement in COVID-19 patients: Risk factors, predictors, and complications: A review, J. Card. Surg,
doi:10.1111/jocs.14538Baig, Khaleeq, Ali, Syeda, Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms, ACS Chem. Neurosci,
doi:10.1021/acschemneuro.0c00122Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps, J. Exp. Med,
doi:10.1084/jem.20200652Brener, Hulke, Fukuma, Golob, Zilinyi et al., Clinico-histopathologic and single-nuclei RNA-sequencing insights into cardiac injury and microthrombi in critical COVID-19, JCI Insight,
doi:10.1172/jci.insight.154633Butowt, Bilinska, SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection, ACS Chem. Neurosci,
doi:10.1021/acschemneuro.0c00172Cao, Li, Feng, Wan, Huang et al., Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations, Cell Discov,
doi:10.1038/s41421-020-0147-1Cenko, Badimon, Bugiardini, Claeys, De Luca et al., Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA), Cardiovasc. Res
Chou, Beghi, Helbok, Moro, Sampson et al., Global Incidence of Neurological Manifestations Among Patients Hospitalized with COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium, JAMA Netw. Open,
doi:10.1001/jamanetworkopen.2021.12131Crivelli, Palmer, Calandri, Guekht, Beghi et al., Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis, Alzheimer's Dement. J. Alzheimer's Assoc,
doi:10.1002/alz.12644De Maistre, Savard, Guinot, COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation, J. Clin. Med,
doi:10.3390/jcm12237245Delorey, Ziegler, Heimberg, Normand, Yang et al., COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets, Nature,
doi:10.1038/s41586-021-03570-8El-Kassas, Alboraie, Elbadry, El Sheemy, Abdellah et al., Non-pulmonary involvement in COVID-19: A systemic disease rather than a pure respiratory infection, World J. Clin. Cases,
doi:10.12998/wjcc.v11.i3.493Fan, Cheek, Chlan, Gosselink, Hart et al., An official American Thoracic Society Clinical Practice guideline: The diagnosis of intensive care unit-acquired weakness in adults, Am. J. Respir. Crit. Care Med,
doi:10.1164/rccm.201411-2011STFernandez-De-Las-Penas, Navarro-Santana, Gomez-Mayordomo, Cuadrado, Garcia-Azorin et al., Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature, Eur. J. Neurol,
doi:10.1111/ene.15040Fullard, Lee, Voloudakis, Suo, Javidfar et al., Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19, Genome Med,
doi:10.1186/s13073-021-00933-8Genovese, Moltrasio, Berti, Marzano, Skin Manifestations Associated with COVID-19: Current Knowledge and Future Perspectives, Dermatology,
doi:10.1159/000512932Giustino, Pinney, Lala, Reddy, Johnston-Cox et al., Myocardial Injury, and Arrhythmia: JACC Focus Seminar, J. Am. Coll. Cardiol,
doi:10.1016/j.jacc.2020.08.059Gonzalez-Gonzalez, Ziccardi, Mccauley, Virchow's Triad and the Role of Thrombosis in COVID-Related Stroke, Front. Physiol,
doi:10.3389/fphys.2021.769254Groff, Kavanaugh, Ramgobin, Mcclafferty, Aggarwal et al., Gastrointestinal Manifestations of COVID-19: A Review of What We Know, Ochsner J,
doi:10.31486/toj.20.0086Hatakeyama, Inoue, Liu, Yamakawa, Nishida et al., Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study, J. Clin. Med,
doi:10.3390/jcm11195758Haussner, Derosa, Haussner, Tran, Torres-Lavoro et al., COVID-19 associated myocarditis: A systematic review, Am. J. Emerg. Med,
doi:10.1016/j.ajem.2021.10.001Herrero-Moyano, Capusan, Andreu-Barasoain, Alcántara-González, Salado et al., A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema, J. Eur. Acad. Dermatol. Venereol,
doi:10.1111/jdv.16631Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell,
doi:10.1016/j.cell.2020.02.052Huang, Lim, Pranata, Diabetesmellitusis associatedwithincreasedmortalityand severityof diseasein COVID-19 pneumonia-A systematicreview, meta-analysis, and meta-regression:diabetesand COVID-19, Diabetes Metab. Syndr. Clin. Res. Rev,
doi:10.1016/j.dsx.2020.04.018Inoue, Hatakeyama, Kondo, Hifumi, Sakuramoto et al., Post-intensive care syndrome: Its pathophysiology, prevention, and future directions, Acute Med. Surg,
doi:10.1002/ams2.415Italia, Tomasoni, Bisegna, Pancaldi, Stretti et al., COVID-19 and Heart Failure: From Epidemiology during the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae, Front. Cardiovasc. Med,
doi:10.3389/fcvm.2021.713560Jin, Lian, Hu, Gao, Zheng et al., Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms, Gut,
doi:10.1136/gutjnl-2020-320926Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes, Cell,
doi:10.1016/j.cell.2020.11.025Katyal, Narula, Acharya, Govindarajan, Neuromuscular Complications with SARS-CoV-2 Infection: A Review, Front. Neurol,
doi:10.3389/fneur.2020.01052Lechien, Chiesa-Estomba, De Siati, Horoi, Le Bon et al., Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study, Eur. Arch. Otorhinolaryngol,
doi:10.1007/s00405-020-05965-1Li, Chen, Zhu, Ben, Gao et al., The impact of ACE2 and co-factors on SARS-CoV-2 infection in colorectal cancer, Clin. Transl. Med,
doi:10.1002/ctm2.967Li, Wang, Chen, Zuo, Zhang et al., COVID-19 infection may cause ketosis and ketoacidosis, Diabetes Obes. Metab,
doi:10.1111/dom.14057Lumbers, Head, Smith, Delforce, Jarrott et al., The interacting physiology of COVID-19 and the renin-angiotensin-aldosterone system: Key agents for treatment, Pharmacol. Res. Perspect,
doi:10.1002/prp2.917Mantovani, Byrne, Zheng, Targher, Diabetes as a risk factorfor greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies, Nutr. Metab. Cardiovasc. Dis,
doi:10.1016/j.numecd.2020.05.014Mao, Jin, Wang, Hu, Chen et al., Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China, JAMA Neurol,
doi:10.1001/jamaneurol.2020.1127Mao, Qiu, He, Tan, Li et al., Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis, Lancet Gastroenterol. Hepatol,
doi:10.1016/S2468-1253(20)30126-6Mariet, Giroud, Benzenine, Cottenet, Roussot et al., Hospitalizations for Stroke in France during the COVID-19 Pandemic Before, During, and After the National Lockdown, Stroke,
doi:10.1161/STROKEAHA.120.032312Martelletti, Bentivegna, Luciani, Spuntarelli, Headache as a Prognostic Factor for COVID-19. Time to Re-evaluate, SN Compr. Clin. Med,
doi:10.1007/s42399-020-00657-7Mehraeen, Behnezhad, Salehi, Noori, Harandi et al., Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence, Eur. Arch. Otorhinolaryngol,
doi:10.1007/s00405-020-06120-6Mehta, Griendling, Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system, Am. J. Physiol. Cell Physiol,
doi:10.1152/ajpcell.00287.2006Miskowiak, Johnsen, Sattler, Nielsen, Kunalan et al., Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables, Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol,
doi:10.1016/j.euroneuro.2021.03.019Morbini, Benazzo, Verga, Pagella, Mojoli et al., Ultrastructural Evidence of Direct Viral Damage to the Olfactory Complex in Patients Testing Positive for SARS-CoV-2, JAMA Otolaryngol.-Head Neck Surg,
doi:10.1001/jamaoto.2020.2366Moslehi, Jahromy, Ashrafi, Vatani, Nemati et al., Multi-organ system involvement in coronavirus disease 2019 (COVID-19): A mega review, J. Fam. Med. Prim. Care,
doi:10.4103/jfmpc.jfmpc_1570_21Muus, Luecken, Eraslan, Sikkema, Waghray et al., Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics, Nat. Med,
doi:10.1038/s41591-020-01227-zMéndez, Balanzá-Martínez, Luperdi, Estrada, Latorre et al., Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors, J. Intern. Med,
doi:10.1111/joim.13262Nanwani-Nanwani, López-Pérez, Giménez-Esparza, Ruiz-Barranco, Carrillo et al., Prevalence of post-intensive care syndrome in mechanically ventilated patients with COVID-19, Sci. Rep,
doi:10.1038/s41598-022-11929-8Nappi, Giacinto, Ellouze, Nenna, Singh et al., Association between COVID-19 Diagnosis and Coronary Artery Thrombosis: A Narrative Review, Biomedicines,
doi:10.3390/biomedicines10030702Palaiodimou, Stefanou, Katsanos, Fragkou, Papadopoulou et al., Prevalence, clinical characteristics and outcomes of Guillain-Barre syndrome spectrum associated with COVID-19: A systematic review and meta-analysis, Eur. J. Neurol,
doi:10.1111/ene.14860Pan, Mu, Yang, Sun, Wang et al., Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study, Am. J. Gastroenterol,
doi:10.14309/ajg.0000000000000620Pan, Xu, Zhang, Zhou, Wang et al., Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis, Intensive Care Med,
doi:10.1007/s00134-020-06026-1Paniri, Hosseini, Akhavan-Niaki, First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations, J. Biomol. Struct. Dyn,
doi:10.1080/07391102.2020.1767690Petito, Falcinelli, Paliani, Cesari, Vaudo et al., Association of Neutrophil Activation, More Than Platelet Activation, with Thrombotic Complications in Coronavirus Disease, J. Infect. Dis,
doi:10.1093/infdis/jiaa756Pimentel, Luchsinger, Carvalho, Alcará, Esper et al., Guillain-Barre syndrome associated with COVID-19: A systematic review, Brain Behav. Immun.-Health,
doi:10.1016/j.bbih.2022.100578Pommier, Benzenine, Bernard, Mariet, Béjot et al., Trends of Myocarditis and Endocarditis Cases before, during, and after the First Complete COVID-19-Related Lockdown in 2020 in France, Biomedicines,
doi:10.3390/biomedicines10061231Puelles, Lutgehetmann, Lindenmeyer, Sperhake, Wong et al., Multiorgan and Renal Tropism of SARS-CoV-2, N. Engl. J. Med,
doi:10.1056/NEJMc2011400Puig-Domingo, Marazuela, Yildiz, Giustina, COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology, Endocrine,
doi:10.1007/s12020-021-02734-wPullen, Skipper, Hullsiek, Bangdiwala, Pastick et al., Symptoms of COVID-19 Outpatients in the United States, Open Forum Infect. Dis,
doi:10.1093/ofid/ofaa271Radermecker, Detrembleur, Guiot, Cavalier, Henket et al., Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19, J. Exp. Med,
doi:10.1084/jem.20201012Reymundo, Fernaldez-Bernaldez, Reolid, Butron, Fernandez-Rico et al., Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients, J. Eur. Acad. Dermatol. Venereol,
doi:10.1111/jdv.16707Sabiescu, Kamal, Kamal, Alexandru, Mitrut, Liver damage in the context of SARS-CoV-2. COVID-19 treatment and its effects on the liver, J. Med. Life,
doi:10.25122/jml-2022-0177Santos, Sampaio, Alzamora, Motta-Santos, Alenina et al., The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7), Physiol. Rev,
doi:10.1152/physrev.00023.2016See, Su, Lale, Woo, Guh et al., US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia after Ad26.COV2.S Vaccination, JAMA,
doi:10.1001/jama.2021.7517Shah, Yasmin, Memon, Jatoi, Savul et al., COVID-19 and myasthenia gravis: A review of neurological implications of the SARS-CoV-2, Brain Behav,
doi:10.1002/brb3.2789Skendros, Mitsios, Chrysanthopoulou, Mastellos, Metallidis et al., Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis, J. Clin. Investig,
doi:10.1172/JCI141374Souza, Buzzetti, Pozzilli, Diabetes, COVID-19, and questions unsolved, Diabetes Metab. Res. Rev,
doi:10.1002/dmrr.3666Stopsack, Mucci, Antonarakis, Nelson, Kantoff, TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention?, Cancer Discov,
doi:10.1158/2159-8290.CD-20-0451Sungnak, Huang, Becavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med,
doi:10.1038/s41591-020-0868-6Theetha Kariyanna, Sabih, Sutarjono, Shah, Vargas Pelaez et al., A Systematic Review of COVID-19 and Pericarditis, Cureus,
doi:10.7759/cureus.27948Tian, Rong, Nian, He, Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission, Aliment. Pharmacol. Ther,
doi:10.1111/apt.15731Unterman, Sumida, Nouri, Yan, Zhao et al., Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19, Nat. Commun,
doi:10.1038/s41467-021-27716-4Veras, Pontelli, Silva, Toller-Kawahisa, De Lima et al., SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology, J. Exp. Med,
doi:10.1084/jem.20201129Vlachopoulos, Aznaouridis, Stefanadis, Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis, J. Am. Coll. Cardiol,
doi:10.1016/j.jacc.2009.10.061Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA,
doi:10.1001/jama.2020.1585Wolff, Nee, Hickey, Marschollek, Risk factors for COVID-19 severity and fatality: A structured literature review, Infection,
doi:10.1007/s15010-020-01509-1Wong, Hu, Zhou, Lloyd, Amend et al., Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: A cohort study in claims databases, Lancet,
doi:10.1016/S0140-6736(22)00791-7Wu, Chen, Cai, Xia, Zhou et al., Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China, JAMA Intern. Med,
doi:10.1001/jamainternmed.2020.0994Wu, Wang, Chen, Ku, Wang, The prevalence of olfactory and gustatory dysfunction in COVID-19-A systematic review, Auris Nasus Larynx,
doi:10.1016/j.anl.2021.07.007Xu, Chu, Zhong, Tan, Tang et al., Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs, Cell Death Discov,
doi:10.1038/s41420-020-00307-wYang, Kern, Losada, Agam, Maat et al., Dysregulation of brain and choroid plexus cell types in severe COVID-19, Nature,
doi:10.1038/s41586-021-03710-0Yang, Lin, Ji, Guo, Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes, Acta Diabetol,
doi:10.1007/s00592-009-0109-4Zanoli, Gaudio, Mikhailidis, Katsiki, Castellino et al., Vascular dysfunction of COVID-19 is partially reverted in the long-term, Circ. Res,
doi:10.1161/CIRCRESAHA.121.320460Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet,
doi:10.1016/S0140-6736(20)30566-3Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med,
doi:10.1056/NEJMoa2001017Zota, Stătescu, Sascău, Roca, Anghel et al., Acute and long-term consequences of COVID-19 on arterial stiffness-A narrative review, Life,
doi:10.3390/life12060781Zou, Chen, Zou, Han, Hao et al., Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection, Front. Med,
doi:10.1007/s11684-020-0754-0DOI record:
{
"DOI": "10.3390/jcm13051397",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm13051397",
"abstract": "<jats:p>First described in December 2019 in Wuhan (China), COVID-19 disease rapidly spread worldwide, constituting the biggest pandemic in the last 100 years. Even if SARS-CoV-2, the agent responsible for COVID-19, is mainly associated with pulmonary injury, evidence is growing that this virus can affect many organs, including the heart and vascular endothelial cells, and cause haemostasis, CNS, and kidney and gastrointestinal tract abnormalities that can impact in the disease course and prognosis. In fact, COVID-19 may affect almost all the organs. Hence, SARS-CoV-2 is essentially a systemic infection that can present a large number of clinical manifestations, and it is variable in distribution and severity, which means it is potentially life-threatening. The goal of this comprehensive review paper in the series is to give an overview of non-pulmonary involvement in COVID-19, with a special focus on underlying pathophysiological mechanisms and clinical presentation.</jats:p>",
"alternative-id": [
"jcm13051397"
],
"author": [
{
"affiliation": [
{
"name": "Department of Neurology, CHU Dijon-Bourgogne, 21000 Dijon, France"
},
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
}
],
"family": "Duloquin",
"given": "Gauthier",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1637-8855",
"affiliation": [
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
},
{
"name": "Department of Cardiology, University Hospital of Dijon, 21000 Dijon, France"
}
],
"authenticated-orcid": false,
"family": "Pommier",
"given": "Thibaut",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pneumology and Intensive Care Unit, Reference Centre for Rare Lung Diseases, Dijon University Hospital, 14 Boulevard Gaffarel, 21000 Dijon, France"
},
{
"name": "Centre des Sciences du Goût et de l’Alimentation, INRA, UMR 6265 CNRS 1234, University of Bourgogne Franche-Comté, 21000 Dijon, France"
}
],
"family": "Georges",
"given": "Marjolaine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurology, CHU Dijon-Bourgogne, 21000 Dijon, France"
},
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
}
],
"family": "Giroud",
"given": "Maurice",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3554-7714",
"affiliation": [
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
},
{
"name": "Department of Cardiology, University Hospital of Dijon, 21000 Dijon, France"
}
],
"authenticated-orcid": false,
"family": "Guenancia",
"given": "Charles",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7848-7072",
"affiliation": [
{
"name": "Department of Neurology, CHU Dijon-Bourgogne, 21000 Dijon, France"
},
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
}
],
"authenticated-orcid": false,
"family": "Béjot",
"given": "Yannick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Cerebro-Vascular Pathophysiology and Epidemiology (PEC2) EA 7460, University of Bourgogne, 21000 Dijon, France"
},
{
"name": "Department of Cardiology, University Hospital of Dijon, 21000 Dijon, France"
}
],
"family": "Laurent",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pneumology and Intensive Care Unit, Reference Centre for Rare Lung Diseases, Dijon University Hospital, 14 Boulevard Gaffarel, 21000 Dijon, France"
}
],
"family": "Rabec",
"given": "Claudio",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T15:37:36Z",
"timestamp": 1709134656000
},
"deposited": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T05:54:25Z",
"timestamp": 1709272465000
},
"indexed": {
"date-parts": [
[
2024,
3,
2
]
],
"date-time": "2024-03-02T00:25:49Z",
"timestamp": 1709339149251
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2024,
2,
28
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2024,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T00:00:00Z",
"timestamp": 1709078400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/13/5/1397/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1397",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
2,
28
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
28
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01509-1",
"article-title": "Risk factors for COVID-19 severity and fatality: A structured literature review",
"author": "Wolff",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Infection",
"key": "ref_2",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.4103/jfmpc.jfmpc_1570_21",
"article-title": "Multi-organ system involvement in coronavirus disease 2019 (COVID-19): A mega review",
"author": "Moslehi",
"doi-asserted-by": "crossref",
"first-page": "5014",
"journal-title": "J. Fam. Med. Prim. Care",
"key": "ref_3",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3390/jcm12237245",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "de Maistre, E., Savard, P., and Guinot, P.G. (2023). COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med., 12."
},
{
"DOI": "10.1038/s41577-020-0343-0",
"article-title": "COVID-19: The vasculature unleashed",
"author": "Teuwen",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_5",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1098/rsob.200208",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Wazny, V., Siau, A., Wu, K.X., and Cheung, C. (2020). Vascular underpinning of COVID-19. Open Biol., 10."
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "JAMA Intern. Med.",
"key": "ref_7",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "ref_8",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_9",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1007/s11684-020-0754-0",
"article-title": "Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Front. Med.",
"key": "ref_10",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-01227-z",
"article-title": "Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics",
"author": "Muus",
"doi-asserted-by": "crossref",
"first-page": "546",
"journal-title": "Nat. Med.",
"key": "ref_11",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1767690",
"article-title": "First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations",
"author": "Paniri",
"doi-asserted-by": "crossref",
"first-page": "3576",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_12",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1038/s41421-020-0147-1",
"article-title": "Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Cell Discov.",
"key": "ref_13",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1152/physrev.00023.2016",
"article-title": "The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7)",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "505",
"journal-title": "Physiol. Rev.",
"key": "ref_14",
"volume": "98",
"year": "2018"
},
{
"DOI": "10.1152/ajpcell.00287.2006",
"article-title": "Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "C82",
"journal-title": "Am. J. Physiol. Cell Physiol.",
"key": "ref_15",
"volume": "292",
"year": "2007"
},
{
"DOI": "10.1002/prp2.917",
"article-title": "The interacting physiology of COVID-19 and the renin-angiotensin-aldosterone system: Key agents for treatment",
"author": "Lumbers",
"doi-asserted-by": "crossref",
"first-page": "e00917",
"journal-title": "Pharmacol. Res. Perspect.",
"key": "ref_16",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.cyto.2020.155151",
"article-title": "COVID-19 cytokine storm: The anger of inflammation",
"author": "Mahmudpour",
"doi-asserted-by": "crossref",
"first-page": "155151",
"journal-title": "Cytokine",
"key": "ref_17",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1002/1096-9896(2000)9999:9999<::AID-PATH743>3.0.CO;2-T",
"article-title": "Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues",
"author": "Vaarala",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "J. Pathol.",
"key": "ref_18",
"volume": "193",
"year": "2001"
},
{
"DOI": "10.1007/s00134-020-06026-1",
"article-title": "Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "1114",
"journal-title": "Intensive Care Med.",
"key": "ref_19",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1158/2159-8290.CD-20-0451",
"article-title": "TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention?",
"author": "Stopsack",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Cancer Discov.",
"key": "ref_20",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-27716-4",
"article-title": "Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19",
"author": "Unterman",
"doi-asserted-by": "crossref",
"first-page": "440",
"journal-title": "Nat. Commun.",
"key": "ref_21",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes",
"author": "Karki",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Cell",
"key": "ref_22",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1084/jem.20200652",
"article-title": "Targeting potential drivers of COVID-19: Neutrophil extracellular traps",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "e20200652",
"journal-title": "J. Exp. Med.",
"key": "ref_23",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa756",
"article-title": "Association of Neutrophil Activation, More Than Platelet Activation, with Thrombotic Complications in Coronavirus Disease 2019",
"author": "Petito",
"doi-asserted-by": "crossref",
"first-page": "933",
"journal-title": "J. Infect. Dis.",
"key": "ref_24",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1186/s12929-022-00872-5",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Borczuk, A.C., and Yantiss, R.K. (2022). The pathogenesis of coronavirus-19 disease. J. Biomed. Sci., 29."
},
{
"DOI": "10.1084/jem.20201012",
"article-title": "Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19",
"author": "Radermecker",
"doi-asserted-by": "crossref",
"first-page": "e20201012",
"journal-title": "J. Exp. Med.",
"key": "ref_26",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1172/JCI141374",
"article-title": "Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis",
"author": "Skendros",
"doi-asserted-by": "crossref",
"first-page": "6151",
"journal-title": "J. Clin. Investig.",
"key": "ref_27",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1084/jem.20201129",
"article-title": "SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology",
"author": "Veras",
"doi-asserted-by": "crossref",
"first-page": "e20201129",
"journal-title": "J. Exp. Med.",
"key": "ref_28",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1186/s13073-021-00933-8",
"article-title": "Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19",
"author": "Fullard",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "Genome Med.",
"key": "ref_29",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1124/molpharm.120.000014",
"article-title": "Does COVID19 Infect the Brain? If So, Smokers Might Be at a Higher Risk",
"author": "Kabbani",
"doi-asserted-by": "crossref",
"first-page": "351",
"journal-title": "Mol. Pharmacol.",
"key": "ref_30",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1021/acschemneuro.0c00122",
"article-title": "Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms",
"author": "Baig",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "ACS Chem. Neurosci.",
"key": "ref_31",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03710-0",
"article-title": "Dysregulation of brain and choroid plexus cell types in severe COVID-19",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Nature",
"key": "ref_32",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1016/j.anl.2021.07.007",
"article-title": "The prevalence of olfactory and gustatory dysfunction in COVID-19—A systematic review",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Auris Nasus Larynx",
"key": "ref_33",
"volume": "49",
"year": "2022"
},
{
"DOI": "10.1001/jamaoto.2020.2366",
"article-title": "Ultrastructural Evidence of Direct Viral Damage to the Olfactory Complex in Patients Testing Positive for SARS-CoV-2",
"author": "Morbini",
"doi-asserted-by": "crossref",
"first-page": "972",
"journal-title": "JAMA Otolaryngol.-Head Neck Surg.",
"key": "ref_34",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1021/acschemneuro.0c00172",
"article-title": "SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection",
"author": "Butowt",
"doi-asserted-by": "crossref",
"first-page": "1200",
"journal-title": "ACS Chem. Neurosci.",
"key": "ref_35",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s42399-020-00657-7",
"article-title": "Headache as a Prognostic Factor for COVID-19. Time to Re-evaluate",
"author": "Martelletti",
"doi-asserted-by": "crossref",
"first-page": "2509",
"journal-title": "SN Compr. Clin. Med.",
"key": "ref_36",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofaa271",
"article-title": "Symptoms of COVID-19 Outpatients in the United States",
"author": "Pullen",
"doi-asserted-by": "crossref",
"first-page": "ofaa271",
"journal-title": "Open Forum Infect. Dis.",
"key": "ref_37",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1111/ene.15040",
"article-title": "Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature",
"author": "Cuadrado",
"doi-asserted-by": "crossref",
"first-page": "3820",
"journal-title": "Eur. J. Neurol.",
"key": "ref_38",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1186/s10194-022-01450-8",
"article-title": "Long COVID headache",
"author": "Tana",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "J. Headache Pain",
"key": "ref_39",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1002/alz.12644",
"article-title": "Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis",
"author": "Crivelli",
"doi-asserted-by": "crossref",
"first-page": "1047",
"journal-title": "Alzheimer’s Dement. J. Alzheimer’s Assoc.",
"key": "ref_40",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1016/j.euroneuro.2021.03.019",
"article-title": "Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables",
"author": "Miskowiak",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol.",
"key": "ref_41",
"volume": "46",
"year": "2021"
},
{
"DOI": "10.1111/joim.13262",
"article-title": "Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors",
"author": "Luperdi",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "J. Intern. Med.",
"key": "ref_42",
"volume": "290",
"year": "2021"
},
{
"DOI": "10.1001/jamaneurol.2020.1127",
"article-title": "Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China",
"author": "Mao",
"doi-asserted-by": "crossref",
"first-page": "683",
"journal-title": "JAMA Neurol.",
"key": "ref_43",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.12131",
"article-title": "Global Incidence of Neurological Manifestations Among Patients Hospitalized with COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium",
"author": "Chou",
"doi-asserted-by": "crossref",
"first-page": "e2112131",
"journal-title": "JAMA Netw. Open",
"key": "ref_44",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.3389/fneur.2020.01052",
"article-title": "Neuromuscular Complications with SARS-CoV-2 Infection: A Review",
"author": "Katyal",
"doi-asserted-by": "crossref",
"first-page": "1052",
"journal-title": "Front. Neurol.",
"key": "ref_45",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fphys.2021.769254",
"article-title": "Virchow’s Triad and the Role of Thrombosis in COVID-Related Stroke",
"author": "Ziccardi",
"doi-asserted-by": "crossref",
"first-page": "769254",
"journal-title": "Front. Physiol.",
"key": "ref_46",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1161/STROKEAHA.120.032312",
"article-title": "Hospitalizations for Stroke in France during the COVID-19 Pandemic Before, During, and After the National Lockdown",
"author": "Mariet",
"doi-asserted-by": "crossref",
"first-page": "1362",
"journal-title": "Stroke",
"key": "ref_47",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.7517",
"article-title": "US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021",
"author": "See",
"doi-asserted-by": "crossref",
"first-page": "2448",
"journal-title": "JAMA",
"key": "ref_48",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1161/STROKEAHA.120.031786",
"article-title": "Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients",
"author": "Qureshi",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Stroke",
"key": "ref_49",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1111/ene.14860",
"article-title": "Prevalence, clinical characteristics and outcomes of Guillain-Barre syndrome spectrum associated with COVID-19: A systematic review and meta-analysis",
"author": "Palaiodimou",
"doi-asserted-by": "crossref",
"first-page": "3517",
"journal-title": "Eur. J. Neurol.",
"key": "ref_50",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.bbih.2022.100578",
"article-title": "Guillain-Barre syndrome associated with COVID-19: A systematic review",
"author": "Pimentel",
"doi-asserted-by": "crossref",
"first-page": "100578",
"journal-title": "Brain Behav. Immun.-Health",
"key": "ref_51",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.3390/jcm11195758",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Hatakeyama, J., Inoue, S., Liu, K., Yamakawa, K., Nishida, T., Ohshimo, S., Hashimoto, S., Kanda, N., Maruyama, S., and Ogata, Y. (2022). Prevalence and Risk Factor Analysis of Post-Intensive Care Syndrome in Patients with COVID-19 Requiring Mechanical Ventilation: A Multicenter Prospective Observational Study. J. Clin. Med., 11."
},
{
"DOI": "10.1038/s41598-022-11929-8",
"article-title": "Prevalence of post-intensive care syndrome in mechanically ventilated patients with COVID-19",
"author": "Carrillo",
"doi-asserted-by": "crossref",
"first-page": "7977",
"journal-title": "Sci. Rep.",
"key": "ref_53",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1164/rccm.201411-2011ST",
"article-title": "An official American Thoracic Society Clinical Practice guideline: The diagnosis of intensive care unit-acquired weakness in adults",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "1437",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_54",
"volume": "190",
"year": "2014"
},
{
"DOI": "10.1002/ams2.415",
"article-title": "Post-intensive care syndrome: Its pathophysiology, prevention, and future directions",
"author": "Inoue",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "Acute Med. Surg.",
"key": "ref_55",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.3389/fneur.2021.607790",
"article-title": "Care for Patients with Neuromuscular Disorders in the COVID-19 Pandemic Era",
"author": "Tseng",
"doi-asserted-by": "crossref",
"first-page": "607790",
"journal-title": "Front. Neurol.",
"key": "ref_56",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/brb3.2789",
"article-title": "COVID-19 and myasthenia gravis: A review of neurological implications of the SARS-CoV-2",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "e2789",
"journal-title": "Brain Behav.",
"key": "ref_57",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/s41591-020-0968-3",
"article-title": "Extrapulmonary manifestations of COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1017",
"journal-title": "Nat. Med.",
"key": "ref_58",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.154633",
"article-title": "Clinico-histopathologic and single-nuclei RNA-sequencing insights into cardiac injury and microthrombi in critical COVID-19",
"author": "Brener",
"doi-asserted-by": "crossref",
"first-page": "e154633",
"journal-title": "JCI Insight",
"key": "ref_59",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03570-8",
"article-title": "COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets",
"author": "Delorey",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Nature",
"key": "ref_60",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1016/j.jacc.2009.10.061",
"article-title": "Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis",
"author": "Vlachopoulos",
"doi-asserted-by": "crossref",
"first-page": "1318",
"journal-title": "J. Am. Coll. Cardiol.",
"key": "ref_61",
"volume": "55",
"year": "2010"
},
{
"DOI": "10.3390/life12060781",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Zota, I.M., Stătescu, C., Sascău, R.A., Roca, M., Anghel, L., Maștaleru, A., Leon-Constantin, M.M., Ghiciuc, C.M., Cozma, S.R., and Dima-Cozma, L.C. (2022). Acute and long-term consequences of COVID-19 on arterial stiffness—A narrative review. Life, 12."
},
{
"DOI": "10.1161/CIRCRESAHA.121.320460",
"article-title": "Vascular dysfunction of COVID-19 is partially reverted in the long-term",
"author": "Zanoli",
"doi-asserted-by": "crossref",
"first-page": "1276",
"journal-title": "Circ. Res.",
"key": "ref_63",
"volume": "130",
"year": "2022"
},
{
"DOI": "10.1161/CIRCRESAHA.118.312563",
"article-title": "Mechanisms of dysfunction in the aging vasculature and role in age-related disease",
"author": "Donato",
"doi-asserted-by": "crossref",
"first-page": "825",
"journal-title": "Circ. Res.",
"key": "ref_64",
"volume": "123",
"year": "2018"
},
{
"DOI": "10.1007/s11883-021-00935-2",
"article-title": "COVID and Cardiovascular Disease: What We Know in 2021",
"author": "Chilazi",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Curr. Atheroscler. Rep.",
"key": "ref_65",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1111/jocs.14538",
"article-title": "Cardiac involvement in COVID-19 patients: Risk factors, predictors, and complications: A review",
"author": "Aghagoli",
"doi-asserted-by": "crossref",
"first-page": "1302",
"journal-title": "J. Card. Surg.",
"key": "ref_66",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.121.056817",
"article-title": "Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis",
"author": "Ammirati",
"doi-asserted-by": "crossref",
"first-page": "1123",
"journal-title": "Circulation",
"key": "ref_67",
"volume": "145",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines10061231",
"doi-asserted-by": "crossref",
"key": "ref_68",
"unstructured": "Pommier, T., Benzenine, E., Bernard, C., Mariet, A.-S., Béjot, Y., Giroud, M., Morgant, M.-C., Steinmetz, E., Guenancia, C., and Bouchot, O. (2022). Trends of Myocarditis and Endocarditis Cases before, during, and after the First Complete COVID-19-Related Lockdown in 2020 in France. Biomedicines, 10."
},
{
"article-title": "A Systematic Review of COVID-19 and Pericarditis",
"author": "Sabih",
"first-page": "e27948",
"journal-title": "Cureus",
"key": "ref_69",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.ajem.2021.10.001",
"article-title": "COVID-19 associated myocarditis: A systematic review",
"author": "Haussner",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Am. J. Emerg. Med.",
"key": "ref_70",
"volume": "51",
"year": "2022"
},
{
"DOI": "10.1161/CIRCIMAGING.121.013771",
"article-title": "Myocarditis Associated With COVID-19 Booster Vaccination",
"author": "Aviram",
"doi-asserted-by": "crossref",
"first-page": "e013771",
"journal-title": "Circ. Cardiovasc. Imaging",
"key": "ref_71",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00791-7",
"article-title": "Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: A cohort study in claims databases",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "2191",
"journal-title": "Lancet",
"key": "ref_72",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1093/cvr/cvab298",
"article-title": "Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA)",
"author": "Cenko",
"doi-asserted-by": "crossref",
"first-page": "2705",
"journal-title": "Cardiovasc. Res.",
"key": "ref_73",
"volume": "117",
"year": "2021"
},
{
"DOI": "10.3390/biomedicines10030702",
"doi-asserted-by": "crossref",
"key": "ref_74",
"unstructured": "Nappi, F., Giacinto, O., Ellouze, O., Nenna, A., Singh, S.S.A., Chello, M., Bouzguenda, A., and Copie, X. (2022). Association between COVID-19 Diagnosis and Coronary Artery Thrombosis: A Narrative Review. Biomedicines, 10."
},
{
"DOI": "10.1016/j.jacc.2020.08.059",
"article-title": "Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar",
"author": "Giustino",
"doi-asserted-by": "crossref",
"first-page": "2011",
"journal-title": "J. Am. Coll. Cardiol.",
"key": "ref_75",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.4414/smw.2020.20417",
"article-title": "Cardiovascular aspects of COVID-19",
"author": "Kurz",
"doi-asserted-by": "crossref",
"first-page": "w20417",
"journal-title": "Swiss Med. Wkly.",
"key": "ref_76",
"volume": "150",
"year": "2020"
},
{
"article-title": "COVID-19 and Cardiac Arrhythmias: A Review of the Literature",
"author": "Akkawi",
"first-page": "e17797",
"journal-title": "Cureus",
"key": "ref_77",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.hrthm.2020.06.016",
"article-title": "COVID-19 and cardiac arrhythmias",
"author": "Bhatla",
"doi-asserted-by": "crossref",
"first-page": "1439",
"journal-title": "Heart Rhythm",
"key": "ref_78",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06555-3",
"article-title": "Cardiac injury in COVID-19",
"author": "Helms",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Intensive Care Med.",
"key": "ref_79",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.3389/fcvm.2021.713560",
"article-title": "COVID-19 and Heart Failure: From Epidemiology during the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae",
"author": "Italia",
"doi-asserted-by": "crossref",
"first-page": "713560",
"journal-title": "Front. Cardiovasc. Med.",
"key": "ref_80",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1111/jgh.15047",
"article-title": "COVID-19 and the digestive system",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "744",
"journal-title": "J. Gastroenterol. Hepatol.",
"key": "ref_81",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2020.107915",
"article-title": "Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection",
"author": "Trottein",
"doi-asserted-by": "crossref",
"first-page": "107915",
"journal-title": "Cell Rep.",
"key": "ref_82",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1111/apt.15731",
"article-title": "Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Aliment. Pharmacol. Ther.",
"key": "ref_83",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1097/JCMA.0000000000000319",
"article-title": "Gastrointestinal and liver manifestations in patients with COVID-19",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "521",
"journal-title": "J. Chin. Med. Assoc. JCMA",
"key": "ref_84",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "ref_85",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.31486/toj.20.0086",
"article-title": "Gastrointestinal Manifestations of COVID-19: A Review of What We Know",
"author": "Groff",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Ochsner J.",
"key": "ref_86",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.12998/wjcc.v11.i3.493",
"article-title": "Non-pulmonary involvement in COVID-19: A systemic disease rather than a pure respiratory infection",
"author": "Alboraie",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "World J. Clin. Cases",
"key": "ref_87",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.14309/ajg.0000000000000620",
"article-title": "Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "Am. J. Gastroenterol.",
"key": "ref_88",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-320926",
"article-title": "Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "1002",
"journal-title": "Gut",
"key": "ref_89",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.02.055",
"article-title": "Evidence for Gastrointestinal Infection of SARS-CoV-2",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "1831",
"journal-title": "Gastroenterology",
"key": "ref_90",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1002/ctm2.967",
"article-title": "The impact of ACE2 and co-factors on SARS-CoV-2 infection in colorectal cancer",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e967",
"journal-title": "Clin. Transl. Med.",
"key": "ref_91",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/s41420-020-00307-w",
"article-title": "Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Cell Death Discov.",
"key": "ref_92",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.25122/jml-2022-0177",
"article-title": "Liver damage in the context of SARS-CoV-2. COVID-19 treatment and its effects on the liver",
"author": "Sabiescu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "J. Med. Life",
"key": "ref_93",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/S2468-1253(20)30126-6",
"article-title": "Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis",
"author": "Mao",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "Lancet Gastroenterol. Hepatol.",
"key": "ref_94",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1515/cclm-2020-0198",
"article-title": "Laboratory abnormalities in patients with COVID-2019 infection",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "1131",
"journal-title": "Clin. Chem. Lab. Med.",
"key": "ref_95",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2020.04.018",
"article-title": "Diabetesmellitusis associatedwithincreasedmortalityand severityof diseasein COVID-19 pneu-monia—A systematicreview, meta-analysis, and meta-regression:diabetesand COVID-19",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "395",
"journal-title": "Diabetes Metab. Syndr. Clin. Res. Rev.",
"key": "ref_96",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.numecd.2020.05.014",
"article-title": "Diabetes as a risk factorfor greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies",
"author": "Mantovani",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "Nutr. Metab. Cardiovasc. Dis.",
"key": "ref_97",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1002/dmrr.3666",
"article-title": "Diabetes, COVID-19, and questions unsolved",
"author": "Buzzetti",
"doi-asserted-by": "crossref",
"first-page": "e3666",
"journal-title": "Diabetes Metab. Res. Rev.",
"key": "ref_98",
"volume": "39",
"year": "2023"
},
{
"DOI": "10.1111/dom.14057",
"article-title": "COVID-19 infection may cause ketosis and ketoacidosis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1935",
"journal-title": "Diabetes Obes. Metab.",
"key": "ref_99",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.2337/diabetes.50.2007.S64",
"article-title": "Beta-cell apoptosis and defense mechanisms: Lessons from type 1 diabetes",
"author": "Eizirik",
"doi-asserted-by": "crossref",
"first-page": "S64",
"journal-title": "Diabetes",
"key": "ref_100",
"volume": "50",
"year": "2001"
},
{
"DOI": "10.1007/s00592-009-0109-4",
"article-title": "Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Acta Diabetol.",
"key": "ref_101",
"volume": "47",
"year": "2010"
},
{
"DOI": "10.1007/s12020-021-02734-w",
"article-title": "COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology",
"author": "Marazuela",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Endocrine",
"key": "ref_102",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1111/j.1523-1755.2005.67130.x",
"article-title": "Acute renal impairment in coronavirus-associated severe acute respiratory syndrome",
"author": "Chu",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Kidney Int.",
"key": "ref_103",
"volume": "67",
"year": "2005"
},
{
"DOI": "10.1016/j.kint.2020.05.006",
"article-title": "Northwell COVID-19 Research Consortium; et al. Acute kidney injury in patients hospitalized with COVID-19",
"author": "Hirsch",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Kidney Int.",
"key": "ref_104",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2011400",
"article-title": "Multiorgan and Renal Tropism of SARS-CoV-2",
"author": "Puelles",
"doi-asserted-by": "crossref",
"first-page": "590",
"journal-title": "N. Engl. J. Med.",
"key": "ref_105",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_106",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Nat. Med.",
"key": "ref_107",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s00405-020-05965-1",
"article-title": "Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study",
"author": "Lechien",
"doi-asserted-by": "crossref",
"first-page": "2251",
"journal-title": "Eur. Arch. Otorhinolaryngol.",
"key": "ref_108",
"volume": "277",
"year": "2020"
},
{
"DOI": "10.1007/s00405-020-06120-6",
"article-title": "Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence",
"author": "Mehraeen",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "Eur. Arch. Otorhinolaryngol.",
"key": "ref_109",
"volume": "278",
"year": "2021"
},
{
"DOI": "10.1159/000512932",
"article-title": "Skin Manifestations Associated with COVID-19: Current Knowledge and Future Perspectives",
"author": "Genovese",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Dermatology",
"key": "ref_110",
"volume": "237",
"year": "2021"
},
{
"DOI": "10.1016/j.abd.2021.08.002",
"article-title": "Skin manifestations associated with COVID-19",
"author": "Seque",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Bras. Dermatol.",
"key": "ref_111",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1111/jdv.16707",
"article-title": "Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients",
"author": "Reymundo",
"doi-asserted-by": "crossref",
"first-page": "e755",
"journal-title": "J. Eur. Acad. Dermatol. Venereol.",
"key": "ref_112",
"volume": "34",
"year": "2020"
},
{
"article-title": "A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema",
"author": "Capusan",
"first-page": "e460",
"journal-title": "J. Eur. Acad. Dermatol. Venereol.",
"key": "ref_113",
"volume": "34",
"year": "2020"
}
],
"reference-count": 113,
"references-count": 113,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/13/5/1397"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Is COVID-19 Infection a Multiorganic Disease? Focus on Extrapulmonary Involvement of SARS-CoV-2",
"type": "journal-article",
"volume": "13"
}