Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial
Jienchi Dorward, Ly-Mee Yu, Gail Hayward, Benjamin R Saville, Oghenekome Gbinigie, Oliver Van Hecke, Emma Ogburn, Philip H Evans, Nicholas Pb Thomas, Mahendra G Patel, Duncan Richards, Nicholas Berry, Michelle A Detry, Christina Saunders, Mark Fitzgerald, Victoria Harris, Milensu Shanyinde, Simon De Lusignan, Monique I Andersson, Christopher C Butler, Fd Richard Hobbs
British Journal of General Practice, doi:10.3399/bjgp.2022.0083
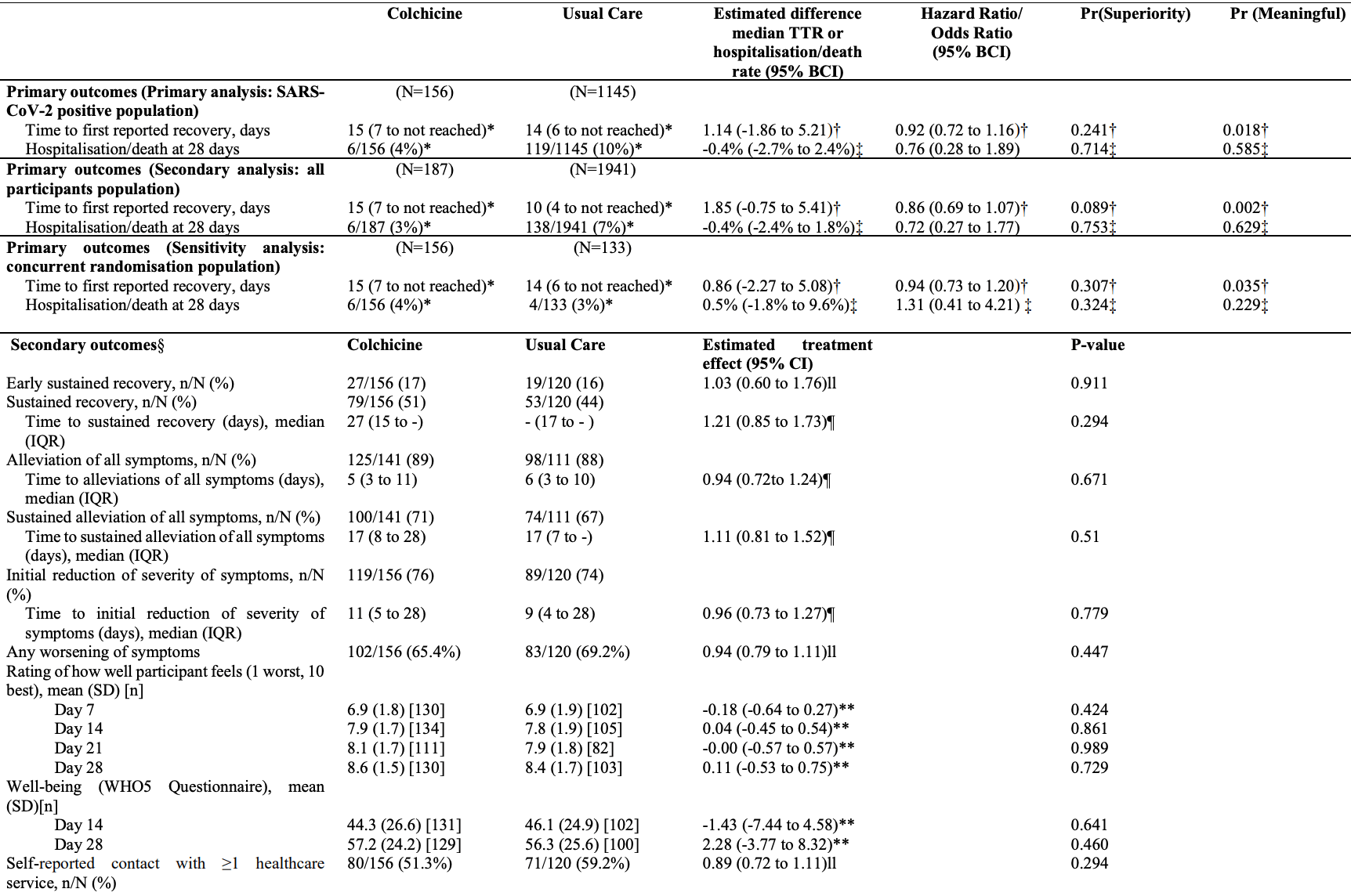
Background: Colchicine has been proposed as a COVID-19 treatment. Aim: To determine whether colchicine reduces time to recovery and COVID-19 related hospitalisations/deaths among people in the community. Design and setting: Prospective, multicentre, open-label, multi-arm, randomised, controlled adaptive platform trial (PRINCIPLE). Method: Adults aged ≥65, or ≥18 years with comorbidities or shortness of breath, and unwell ≤14 days with suspected COVID-19 in the community were randomised to usual care, usual care plus colchicine (500µg daily for 14 days), or usual care plus other interventions. The co-primary endpoints were time to first self-reported recovery, and hospitalisation/death related to COVID-19, within 28 days, analysed using Bayesian models. The hypothesis for the time to recovery endpoint is evaluated first, and if superiority is declared on time to recovery, the hypothesis for the second co-primary endpoint of hospitalisation/death is then evaluated. To determine futility, we pre-specified a clinically meaningful benefit in time to first reported recovery as a hazard ratio of 1.2 or larger (equating to approximately 1.5 days benefit in the colchicine arm, assuming 9 days recovery in the usual care arm).
Strengths include the pragmatic design of the PRINCIPLE trial which allowed for efficient evaluation of the effectiveness of colchicine as an early, standalone intervention as it might be used in the community. We focused on patients at increased risk of complications and used routine electronic health records to confirm hospitalisation/death, and obtained primary outcome data on over 95% of participants. Although our primary analysis was restricted to SARS-CoV-2 positive patients, we conducted secondary analyses of the co-primary outcomes among patients with suspected COVID-19 but without PCR confirmed SARS-CoV-2 infection, as limited SARS-CoV-2 testing may necessitate early empirical treatment in low resource settings. Furthermore, variation in PCR testing sensitivity, particularly if self-administered, means some participants will have had false negative tests.( 28 ) Time to recovery estimates were similar in the SARS-CoV-2 positive population, all participants irrespective of SARS-CoV-2 status, as well as the concurrent randomisation SARS-CoV-2 positive population (the latter populations are most analogous to those in traditional two arm trials). Although the sample size of the colchicine group in PRINCIPLE was relatively small, the Bayesian primary analysis model leverages previous enrolments in the usual care arm to increase the precision of estimates, which allow us to declare futility with high precision due to very low probability of a meaningful benefit of..
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Butler, Dorward, Yu, Gbinigie, Hayward et al., Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet
Butler, Yu, Dorward, Gbinigie, Hayward et al., Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet Respiratory Medicine
Dalbeth, Lauterio, Wolfe, Mechanism of action of colchicine in the treatment of gout
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Network Open
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Horby, Campbell, Spata, Emberson, Staplin et al., Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med
Lopes, Bonjorno, Giannini, Amaral, Menezes et al., Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open
Patel, Dorward, Yu, Hobbs, Butler, Inclusion and diversity in the PRINCIPLE trial, The Lancet
Reyes, Hu, Teperman, Wampler Muskardin, Tardif et al., Anti-inflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med
Rodriguez-Nava, Garcia, Yanez-Bello, Chung, Garcia et al., Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study, Critical Care
Scarsi, Piantoni, Colombo, Airo, Richini et al., Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis
Shi, Wang, Wang, Duan, Yang, Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19, J Infect
Skipper, Pastick, Engen, Bangdiwala, Abassi et al., Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial, Ann Intern Med
Tardif, Bouabdallaoui, Allier, Gaudet, Shah et al., Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med
Topp, Østergaard, Søndergaard, Bech, The WHO-5 Well-Being Index: A Systematic Review of the Literature, Psychotherapy and Psychosomatics
Verity, Okell, Dorigatti, Winskill, Whittaker et al., Estimates of the severity of coronavirus disease 2019: a model-based analysis, The Lancet Infectious Diseases
Woloshin, Patel, Kesselheim, False Negative Tests for SARS-CoV-2 Infection -Challenges and Implications, New England Journal of Medicine
Woodcock, Lavange, Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both, N Engl J Med
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial
DOI record:
{
"DOI": "10.3399/bjgp.2022.0083",
"ISSN": [
"0960-1643",
"1478-5242"
],
"URL": "http://dx.doi.org/10.3399/bjgp.2022.0083",
"abstract": "<jats:p>Background: Colchicine has been proposed as a COVID-19 treatment. Aim: To determine whether colchicine reduces time to recovery and COVID-19 related hospitalisations/deaths among people in the community. Design and setting: Prospective, multicentre, open-label, multi-arm, randomised, controlled adaptive platform trial (PRINCIPLE). Method: Adults aged ≥65, or ≥18 years with comorbidities or shortness of breath, and unwell ≤14 days with suspected COVID-19 in the community were randomised to usual care, usual care plus colchicine (500μg daily for 14 days), or usual care plus other interventions. The co-primary endpoints were time to first self-reported recovery, and hospitalisation/death related to COVID-19, within 28 days, analysed using Bayesian models. The hypothesis for the time to recovery endpoint is evaluated first, and if superiority is declared on time to recovery, the hypothesis for the second co-primary endpoint of hospitalisation/death is then evaluated. To determine futility, we pre-specified a clinically meaningful benefit in time to first reported recovery as a hazard ratio of 1.2 or larger (equating to approximately 1.5 days benefit in the colchicine arm, assuming 9 days recovery in the usual care arm). Results: The trial opened on April 2, 2020, with randomisation to colchicine from March 04, 2021 until May 26, 2021, after the pre-specified time to recovery futility criterion was met. The primary analysis model included 2755 SARS-CoV-2 positive participants, randomised to colchicine (n=156), usual care (n=1145), and other treatments (n=1454). Time to first self-reported recovery was similar in the colchicine group compared with usual care with an estimated hazard ratio of 0·919 [95% credible interval 0·72 to 1·16] and an estimated increase of 1.4 days in median time to self-reported recovery for colchicine versus usual care. The probability of meaningful benefit in time to recovery was very low at 1.8%. Results were similar in comparisons with concurrent controls. COVID-19 related hospitalisations/deaths were similar in the colchicine group versus usual care, with an estimated odds ratio of 0·76 [0·28 to 1·89] and an estimated difference of 0.4% [-2.4 to 2.7%]. One serious adverse event occurred in the colchicine group and two in usual care. Conclusions: Colchicine did not improve time to recovery in people at higher risk of complications with COVID-19 in the community. Trial registration: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"isrctn\" xlink:href=\"86534580\">ISRCTN86534580</jats:ext-link>.</jats:p>",
"alternative-id": [
"10.3399/BJGP.2022.0083"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6072-1430",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dorward",
"given": "Jienchi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yu",
"given": "Ly-mee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0852-627X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hayward",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saville",
"given": "Benjamin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2963-4491",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gbinigie",
"given": "Oghenekome Abisoye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Hecke",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogburn",
"given": "Emma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5277-3545",
"affiliation": [],
"authenticated-orcid": false,
"family": "Evans",
"given": "Philip Hugh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahendra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richards",
"given": "Duncan B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Christina T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detry",
"given": "Michelle A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shanyinde",
"given": "Milensu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8553-2641",
"affiliation": [],
"authenticated-orcid": false,
"family": "de Lusignan",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andersson",
"given": "Monique I",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0102-3453",
"affiliation": [],
"authenticated-orcid": false,
"family": "Butler",
"given": "Christopher C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7976-7172",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hobbs",
"given": "FD Richard",
"sequence": "additional"
}
],
"container-title": [
"British Journal of General Practice"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
23
]
],
"date-time": "2022-03-23T18:25:12Z",
"timestamp": 1648059912000
},
"deposited": {
"date-parts": [
[
2022,
3,
23
]
],
"date-time": "2022-03-23T18:25:12Z",
"timestamp": 1648059912000
},
"indexed": {
"date-parts": [
[
2022,
3,
23
]
],
"date-time": "2022-03-23T18:42:35Z",
"timestamp": 1648060955048
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0960-1643"
},
{
"type": "electronic",
"value": "1478-5242"
}
],
"issued": {
"date-parts": [
[
2022,
3,
23
]
]
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.3399/BJGP.2022.0083",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1987",
"original-title": [],
"page": "BJGP.2022.0083",
"prefix": "10.3399",
"published": {
"date-parts": [
[
2022,
3,
23
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
23
]
]
},
"publisher": "Royal College of General Practitioners",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://bjgp.org/lookup/doi/10.3399/BJGP.2022.0083"
}
},
"score": 1,
"short-container-title": [
"Br J Gen Pract"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Family Practice"
],
"subtitle": [],
"title": [
"Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial"
],
"type": "journal-article"
}