First COVID-19 wave in the province of Bergamo, Italy: epidemiological and clinical characteristics, outcome and management of the first hospitalized patients
Bianca Maria Donida, Flavia Simonetta Pirola, Roberto Opizzi, Peter Assembergs
BMC Infectious Diseases, doi:10.1186/s12879-024-09034-4
Background Northern Italy was the first European country affected by the spread of the SARS-CoV-2, with the epicenter in the province of Bergamo. Aim This study aims to analyze the characteristics of patients who experienced more severe symptoms during the first wave of COVID-19 pandemic.
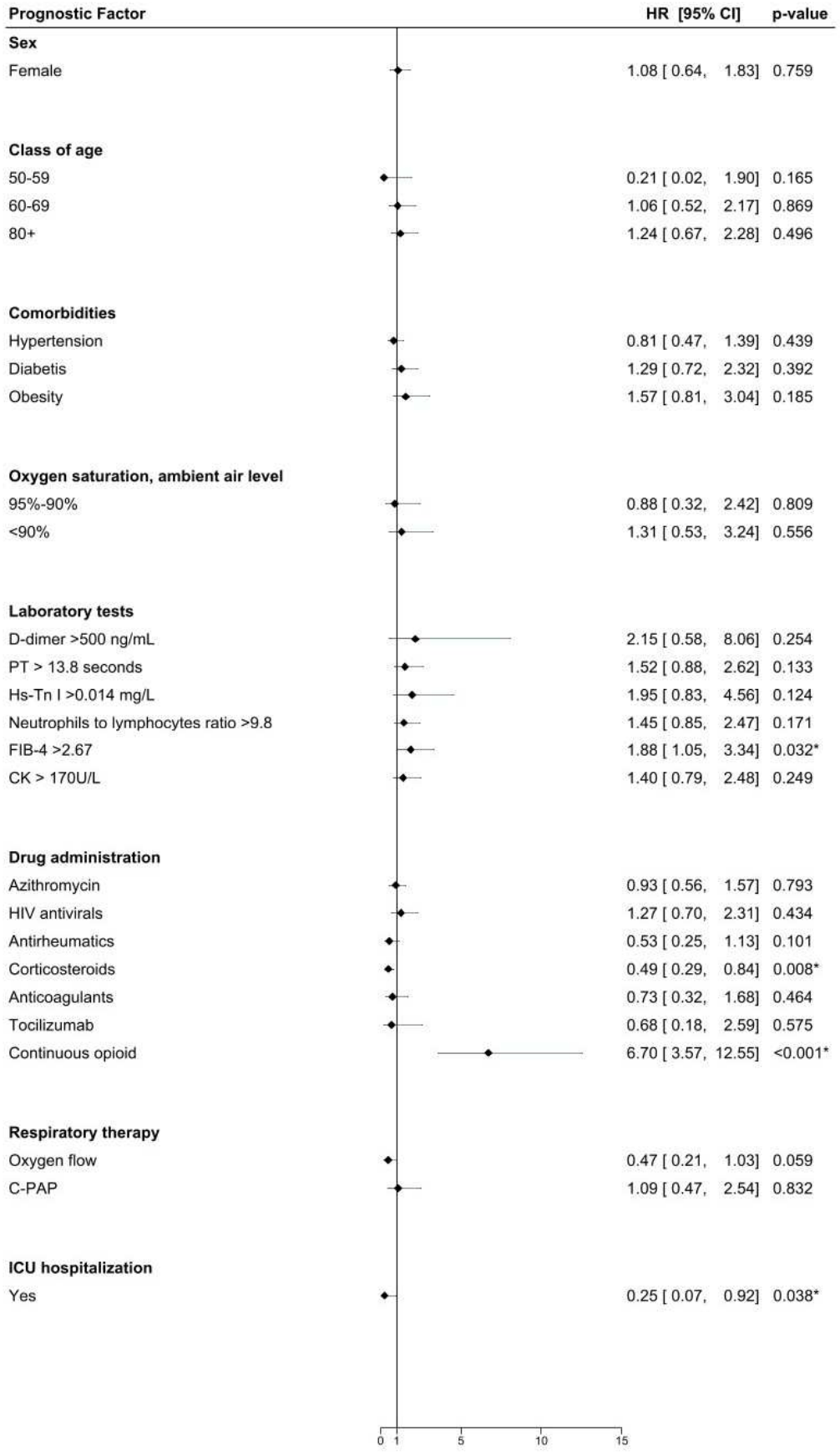
Materials and methods We retrospectively collected epidemiological and clinical data on patients with laboratoryconfirmed wild-type SARS-CoV-2 infection who were admitted to the "ASST Bergamo Ovest" hospital between February 21 and May 31, 2020. Results A total of seven hundred twenty-three inpatients met the eligible criteria and were included in the study cohort. Among the inpatients who survived, the average hospital length of stay was more than two weeks, with some lasting up to three months. Among the 281 non-survivors, death occurred in 50% within five days. Survivors were those whose first aid operators recorded higher oxygen saturation levels at home. The request for first aid assistance came more than one week after symptom onset, within three days in 10% of cases.
Conclusion In similar future scenarios, based on our data, if we aim to enhance the survival rate, we need to improve the territorial healthcare assistance and admit to hospitals only those patients who are at risk of severe illness requiring specialized and urgent interventions within two, three, or, at most, five days from the onset of symptoms. This implies that the crucial factor is, has been, and will be the ability of a healthcare system to react promptly in its entirety within a few days.
Abbreviations
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12879-024-09034-4.
Supplementary Material 1 Author contributions PA defined the area of interest, and he delineated the research's project. BMD organized the work, investigated hospital discharge records, medical charts, and pathological reports, manually implementing the database. RO extracted
Declarations Ethics approval and consent to participate This study was submitted to and approved by the Ethics Committee of Bergamo (Bergamo, BG, Italy) with the procedure's reference "096/22". All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s) in accordance with the approved ethical standards for retrospective observational study without drugs.
Consent for publication Not Applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Cattelan, Meco, Clinical characteristics and laboratory biomarkers changes in COVID-19 patients requiring or not intensive or sub-intensive care: a comparative study, BMC Infectious Diseases
Collaborators, Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality 2020-21, The Lancet
Control, Case definition for coronavirus disease 2019
Elshazli, Toraih, Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients, PLoSONE
Ibanez-Samaniego, Bighelli, Elevation of liver fibrosis index FIB-4 is associated with poor clinical outcomes in patients with COVID-19, J infect Dis,
doi:10.1093/infdis/jiaa355Izcovich, Ragusa, Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review, PLoSONE
Kermali, Khalsa, The role of biomarkers in diagnosis of COVID-19-A systematic review, Life Sciences
Lee, Mayberry, Crapo, Jensen, The accuracy of pulse oximetry in the emergency department, Am J Emerg Med,
doi:10.1053/ajem.2000.7330Ma, Cheng, Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients, Crit Care
Marin, Aghagoli, Predictors of COVID-19 severity: a literature review, Rev Med Virol
Ministry, Health, Report Vaccini Anti COVID-19
Pdw, Fumeaux, Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort, EClinicalMedicine
Riccioni, Raccomandazioni Di Etica Clinica per l'ammissione a trattamenti intensivi e per la loro sospensione, in condizioni eccezionali di squilibrio tra necessità e risorse disponibili, recenti prog Med,
doi:10.1701/3347.33183DOI record:
{
"DOI": "10.1186/s12879-024-09034-4",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-09034-4",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Northern Italy was the first European country affected by the spread of the SARS-CoV-2, with the epicenter in the province of Bergamo.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Aim</jats:title>\n <jats:p>This study aims to analyze the characteristics of patients who experienced more severe symptoms during the first wave of COVID-19 pandemic.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Materials and methods</jats:title>\n <jats:p>We retrospectively collected epidemiological and clinical data on patients with laboratory-confirmed wild-type SARS-CoV-2 infection who were admitted to the “ASST Bergamo Ovest” hospital between February 21 and May 31, 2020.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of seven hundred twenty-three inpatients met the eligible criteria and were included in the study cohort. Among the inpatients who survived, the average hospital length of stay was more than two weeks, with some lasting up to three months. Among the 281 non-survivors, death occurred in 50% within five days. Survivors were those whose first aid operators recorded higher oxygen saturation levels at home. The request for first aid assistance came more than one week after symptom onset, within three days in 10% of cases.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>In similar future scenarios, based on our data, if we aim to enhance the survival rate, we need to improve the territorial healthcare assistance and admit to hospitals only those patients who are at risk of severe illness requiring specialized and urgent interventions within two, three, or, at most, five days from the onset of symptoms. This implies that the crucial factor is, has been, and will be the ability of a healthcare system to react promptly in its entirety within a few days.</jats:p>\n </jats:sec>",
"alternative-id": [
"9034"
],
"article-number": "151",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 January 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "19 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "31 January 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was submitted to and approved by the Ethics Committee of Bergamo (Bergamo, BG, Italy) with the procedure’s reference “096/22”. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s) in accordance with the approved ethical standards for retrospective observational study without drugs."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not Applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Donida",
"given": "Bianca Maria",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pirola",
"given": "Flavia Simonetta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Opizzi",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assembergs",
"given": "Peter",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
31
]
],
"date-time": "2024-01-31T08:03:39Z",
"timestamp": 1706688219000
},
"deposited": {
"date-parts": [
[
2024,
1,
31
]
],
"date-time": "2024-01-31T08:05:56Z",
"timestamp": 1706688356000
},
"indexed": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:38:33Z",
"timestamp": 1706747913159
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
31
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
31
]
],
"date-time": "2024-01-31T00:00:00Z",
"timestamp": 1706659200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
31
]
],
"date-time": "2024-01-31T00:00:00Z",
"timestamp": 1706659200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09034-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-09034-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09034-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
1,
31
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
31
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "9034_CR1",
"unstructured": "World Health Organization., «Coronavirus - Situation report 1,» 21 Jan 2020. [Online]. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf. [Accessed Dec 2022]."
},
{
"key": "9034_CR2",
"unstructured": "World Health Organization, «Report of the WHO-China Joint Mission on Coronavirus Disease. 2019 (COVID-19)» 16–24 February 2020. [Online]. Available: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. [Accessed Dec 2022]."
},
{
"key": "9034_CR3",
"unstructured": "World Health Organization., «Coronavirus - Situation report 31» 20 Feb 2020. [Online]. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200220-sitrep-31-covid-19.pdf?sfvrsn=dfd11d24_2. [Accessed Dec 2022]."
},
{
"key": "9034_CR4",
"unstructured": "World Health Organization., «WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020» WHO, 11 Mar 2020. [Online]. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [Accessed Dec 2022]."
},
{
"key": "9034_CR5",
"unstructured": "Collaborators COVID-. 19 Excess Mortality, «Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality 2020–21» The Lancet, Vol. %1 di %2S0140-6736, n. 21, pp. 02796-3, 10 Mar 2022."
},
{
"key": "9034_CR6",
"unstructured": "Italian Ministry of Health., «Report Vaccini Anti COVID-19» 11 Apr 2022. [Online]. Available: https://www.governo.it/it/cscovid19/report-vaccini/. [Accessed Dec 2022]."
},
{
"DOI": "10.15557/PiMR.2020.0003",
"doi-asserted-by": "crossref",
"key": "9034_CR7",
"unstructured": "World Health Organization., «Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected» 13 Mar 2020. [Online]. Available: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. [Accessed Dec 2022]."
},
{
"key": "9034_CR8",
"unstructured": "World Health Organization., «Clinical management of Covid - Interim Guidance» 27 May 2020. [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf?sequence=1&isAllowed=y. [Accessed Dec 2022]."
},
{
"key": "9034_CR9",
"unstructured": "European Centre for Disease Prevention and Control., «Case definition for coronavirus disease 2019 (COVID-19)» 2020, 20 Dec. [Online]. Available: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition. [Accessed Dec 2022]."
},
{
"key": "9034_CR10",
"unstructured": "European Union, «REGULATION, EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL» 27 Apr. (2016. [Online]. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679&from=EN. [Accessed Dec 2022]."
},
{
"key": "9034_CR11",
"unstructured": "Agenzia Italiana del Farmaco., «Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia (TOCIVID-19)» 18 Mar 2020. [Online]. Available: https://www.aifa.gov.it/documents/20142/1161351/TOCIVID-19_documenti.zip?t=1589463488981&download=true. [Accessed Dec 2022]."
},
{
"DOI": "10.1002/rmv.2146",
"author": "BG Marin",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Rev Med Virol",
"key": "9034_CR12",
"unstructured": "Marin BG, Aghagoli G, et al. «Predictors of COVID-19 severity: a literature review». Rev Med Virol. 2021;31(1):1–10.",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2020.117788",
"doi-asserted-by": "crossref",
"key": "9034_CR13",
"unstructured": "Kermali M, Khalsa RK et al. «The role of biomarkers in diagnosis of COVID-19– A systematic review» Life Sciences, vol. 254, n. 117788, 2020."
},
{
"DOI": "10.1371/journal.pone.0241955",
"doi-asserted-by": "crossref",
"key": "9034_CR14",
"unstructured": "Izcovich A, Ragusa MA et al. «Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review» PLoSONE, vol. 15, n. 11, p. e0241955, 2020."
},
{
"DOI": "10.1371/journal.pone.0238160",
"doi-asserted-by": "crossref",
"key": "9034_CR15",
"unstructured": "Elshazli RM, Toraih EA et al. «Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients» PLoSONE, vol. 15, n. 8, p. e0238160, 2020."
},
{
"DOI": "10.1186/s12879-020-05647-7",
"doi-asserted-by": "crossref",
"key": "9034_CR16",
"unstructured": "Cattelan AM, Di Meco E et al. «Clinical characteristics and laboratory biomarkers changes in COVID-19 patients requiring or not intensive or sub-intensive care: a comparative study» BMC Infectious Diseases, vol. 20, n. 934, 2020."
},
{
"DOI": "10.1016/j.eclinm.2020.100449",
"author": "T Fumeaux",
"doi-asserted-by": "publisher",
"first-page": "100449",
"journal-title": "EClinicalMedicine",
"key": "9034_CR17",
"unstructured": "PDW, Fumeaux T, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449.",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1164/rccm.167.2.211",
"author": "American Thoracic Society",
"doi-asserted-by": "crossref",
"first-page": "211",
"issue": "2",
"journal-title": "Am J Respir Crit Care Med",
"key": "9034_CR18",
"unstructured": "American Thoracic Society. American College of Chest Physicians. «ATS/ACCP Statement on cardiopulmonary». Am J Respir Crit Care Med. 2003;167(2):211–77.",
"volume": "167",
"year": "2003"
},
{
"DOI": "10.1053/ajem.2000.7330",
"doi-asserted-by": "publisher",
"key": "9034_CR19",
"unstructured": "Lee WW, Mayberry K, Crapo R, Jensen RL. «The accuracy of pulse oximetry in the emergency department» Am J Emerg Med. 2000;18(4):427– 31. https://doi.org/10.1053/ajem.2000.7330. PMID: 10919532."
},
{
"key": "9034_CR20",
"unstructured": "Italian Ministry of Health., «Sorveglianza Integrata COVID-19 in Italia» 29 May 2020. [Online]. Available: https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_29maggio%20ITA.pdf. [Accessed Dec 2022]."
},
{
"DOI": "10.1186/s13054-020-03007-0",
"doi-asserted-by": "crossref",
"key": "9034_CR21",
"unstructured": "Ma A, Cheng J et al. «Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients» Crit Care, vol. 24, n. 1, p. 288, 2020."
},
{
"DOI": "10.1093/infdis/jiaa355",
"doi-asserted-by": "crossref",
"key": "9034_CR22",
"unstructured": "Ibanez-Samaniego L, Bighelli F et al. «Elevation of liver fibrosis index FIB-4 is associated with poor clinical outcomes in patients with COVID-19» J infect Dis., p. 10.1093/ infdis/jiaa355, 2020."
},
{
"DOI": "10.1701/3347.33183",
"doi-asserted-by": "publisher",
"key": "9034_CR23",
"unstructured": "Riccioni L et al. «Raccomandazioni Di Etica Clinica per l’ammissione a trattamenti intensivi e per la loro sospensione, in condizioni eccezionali di squilibrio tra necessità e risorse disponibili» recenti prog Med 2020, n. 111, pp. 207–11. https://doi.org/10.1701/3347.33183, 2020."
},
{
"key": "9034_CR24",
"unstructured": "World Health Organization., «Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance,» 28 Jan 2020. [Online]. Available: https://apps.who.int/iris/handle/10665/330893. [Accessed Dec 2022]."
},
{
"key": "9034_CR25",
"unstructured": "Società Italiana di Malattie Infettive Tropicali., «Linee guida sulla gestione terapeutica e di supporto per pazienti con infezione da coronavirus COVID-19» Mar 2020. [Online]. Available: https://www.simit.org/images/regioni/lombardia/comunicazioni/Vademecum%20COVID-19/4.%20vademecum%201.0%20%2001.03.20.pdf. [Accessed Dec 2022]."
},
{
"key": "9034_CR26",
"unstructured": "Agenzia Italiana del Farmaco., «Schede informative sui farmaci utilizzati per emergenza COVID-19 e relative modalità di prescrizione» [Online]. Available: https://www.aifa.gov.it/web/guest/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19. [Accessed Dec 2022]."
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09034-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "First COVID-19 wave in the province of Bergamo, Italy: epidemiological and clinical characteristics, outcome and management of the first hospitalized patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}