What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study
Andrea De Vito, Agnese Colpani, Mariacristina Poliseno, Lucia Diella, Francesco Rosario Paolo Ieva, Alessandra Belati, Roberto Papale, Sergio Babudieri, Laura De Santis, Annalisa Saracino, Sergio Lo Caputo, Giordano Madeddu
Viruses, doi:10.3390/v15081757
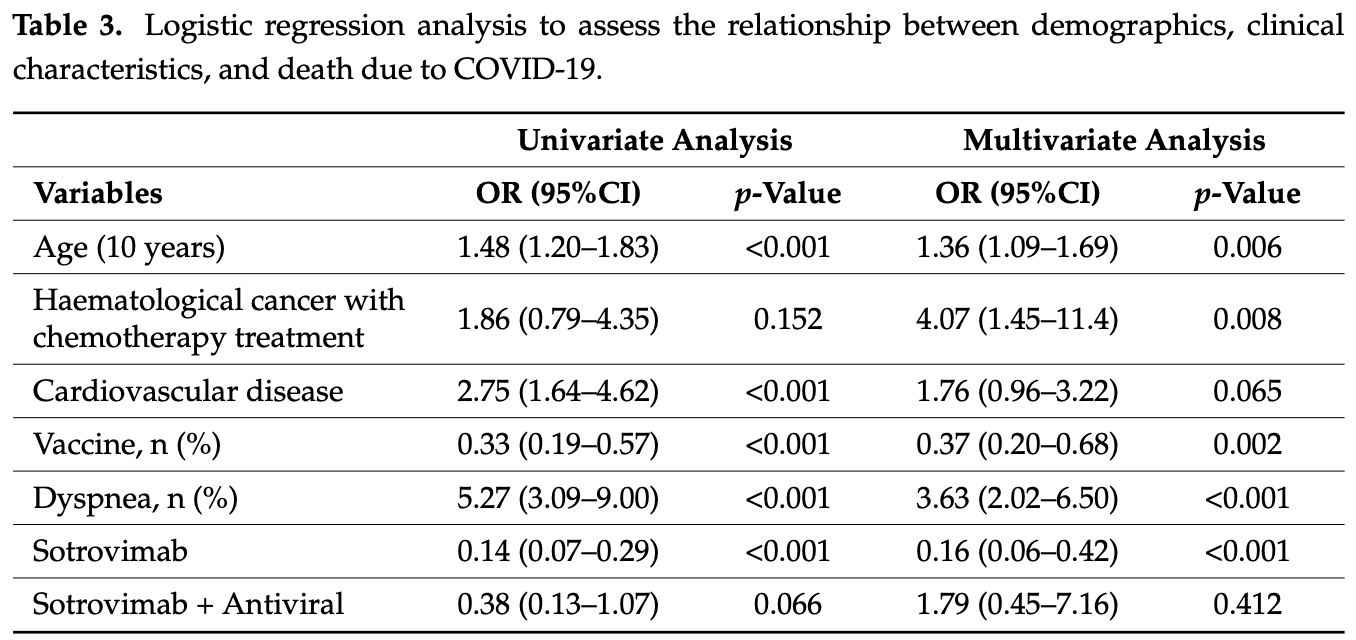
1) Introduction: Since May 2021, sotrovimab has been available in Italy for early treatment of SARS-CoV-2 infection and to prevent disease progression. However, some in vitro studies have questioned its efficacy on Omicron variants. Therefore, we aim to further investigate the efficacy of sotrovimab in real-life settings. (2) Methods: We conducted a retrospective study collecting medical records of people with SARS-CoV-2 infection evaluated in the infectious diseases units of Sassari, Foggia, and Bari, Italy. We included people with SARS-CoV-2 infection treated with sotrovimab and people who did not receive any treatment in 2022. The primary study outcome was to evaluate the efficacy of sotrovimab in reducing disease progression (defined as the necessity of starting oxygen supplementation) and COVID-19-related death. The secondary outcome was to evaluate the safety of sotrovimab. (3) Results: We included 689 people; of them, 341 were treated with sotrovimab, while 348 did not receive any treatment. Overall, we registered 161 (23.4%) disease progressions and 65 (9.4%) deaths, with a significant difference between treated and not-treated people (p < 0.001). In the multivariate logistic regression, increasing age [OR for ten years increasing age 1.23 (95%CI 1.04-1.45)] was associated with a higher risk of disease progression. In addition, cardiovascular disease [OR 1.69 (1.01-2.80), fever )], and dyspnea [OR 7.24 (95%CI 4.17-12.58)] were associated with an increased risk of disease progression. In contrast, vaccination [OR 0.21 (95%CI 0.12-0.37)] and sotrovimab administration [OR 0.05 (95%CI 0.02-0.11)] were associated with a lower risk of developing severe COVID-19. Regarding mortality, people with older age [OR for ten years increasing age 1.36 (95%CI 1.09-1.69)] had a higher risk of death. In addition, in the multivariate analysis, cardiovascular disease lost statistical significance, while people on chemotherapy for haematological cancer )] and those with dyspnea at diagnosis [OR 3.63 (95%CI 2.02-6.50)] had an increased risk of death. In contrast, vaccination ] and sotrovimab treatment [OR 0.16 (95%CI 0.06-0.42)] were associated with lower risk. Only two adverse events were reported; one person complained of diarrhoea a few hours after sotrovimab administration, and one had an allergic reaction with cutaneous rash and itching. (4) Conclusions: Our study showed that sotrovimab treatment was associated with a reduction of the risk of disease progression and death in SARS-CoV-2-infected people, 70% of whom were over 65 years and a with high vaccination rate, with excellent safety. Therefore, our results reinforce the evidence about the efficacy and safety of sotrovimab during the Omicron era in a real-world setting.
Conflicts of Interest: G.M. has been advisor for Gilead Sciences, ViiV, and MSD and has received speakers' honoraria from Gilead Sciences, ViiV, MSD, and GSK. A.S. has been advisor for Gilead Sciences, ViiV, Janssen, Astrazeneca, GSK, and MSD and has received speakers' honoraria from Gilead Sciences, ViiV, M.S.D., Janssen, Astrazeneca, and GSK. S.L.C. has been advisor for Gilead Sciences and GSK and has received speakers' honoraria from Gilead Sciences and GSK. The other authors declare no conflict of interest.
References
Aggarwal, Beaty, Bennett, Carlson, Davis et al., Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients, J,
doi:10.1093/infdis/jiac206Araf, Akter, Tang, Fatemi, Parvez et al., Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines, J. Med. Virol,
doi:10.1002/jmv.27588Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis,
doi:10.1016/S2666-5247(20)30172-5Chan, Kok, Zhu, Chu, To et al., Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan, Emerg. Microbes Infect,
doi:10.1080/22221751.2020.1719902Chavarot, Melenotte, Amrouche, Rouzaud, Sberro-Soussan et al., Early Treatment with Sotrovimab Monoclonal Antibody in Kidney Transplant Recipients with Omicron Infection, Kidney Int,
doi:10.1016/j.kint.2022.04.003Chenchula, Karunakaran, Sharma, Chavan, Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review, J. Med. Virol,
doi:10.1002/jmv.27697Cheng, Reyes, Satram, Birch, Gibbons et al., Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA, Infect. Dis. Ther,
doi:10.1007/s40121-022-00755-0De Vito, Colpani, Bitti, Zauli, Meloni et al., Safety and Efficacy of Molnupiravir in SARS-CoV-2 Infected Patients: A Real-Life Experience, J. Med. Virol,
doi:10.1002/jmv.28011De Vito, Colpani, Madeddu, Shedding New Light on COVID-19 Therapeutics during the Omicron Era: A Deeper Dive into Real-World Data, Lancet Reg. Health Eur,
doi:10.1016/j.lanepe.2023.100694De Vito, Colpani, Saderi, Puci, Zauli et al., Impact of Early SARS-CoV-2 Antiviral Therapy on Disease Progression, Viruses,
doi:10.3390/v15010071De Vito, Fiore, Princic, Geremia, Panu Napodano et al., Predictors of Infection, Symptoms Development, and Mortality in People with SARS-CoV-2 Living in Retirement Nursing Homes, PLoS ONE,
doi:10.1371/journal.pone.0248009De Vito, Geremia, Fiore, Princic, Babudieri et al., Clinical Features, Laboratory Findings and Predictors of Death in Hospitalized Patients with COVID-19 in Sardinia, Italy, Eur. Rev. Med. Pharmacol. Sci
Drake, Riad, Fairfield, Egan, Knight et al., Characterisation of In-Hospital Complications Associated with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol UK: A Prospective, Multicentre Cohort Study, Lancet,
doi:10.1016/S0140-6736(21)00799-6Duong, Alpha, Beta, Gamma: What's Important to Know about SARS-CoV-2 Variants of Concern?, CMAJ Can. Med. Assoc. J,
doi:10.1503/cmaj.1095949Evans, Qi, Adebayo, Underwood, Coulson et al., Real-World Effectiveness of Molnupiravir, Nirmatrelvir-Ritonavir, and Sotrovimab on Preventing Hospital Admission among Higher-Risk Patients with COVID-19 in Wales: A Retrospective Cohort Study, J. Infect,
doi:10.1016/j.jinf.2023.02.012Geremia, De Vito, Gunnella, Fiore, Princic et al., A Case of Vasculitis-Like Skin Eruption Associated With COVID-19, Infect. Dis. Clin,
doi:10.1097/IPC.0000000000000952Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N. Engl. J. Med,
doi:10.1056/NEJMoa2107934Hoffmann, Krüger, Schulz, Cossmann, Rocha et al., The Omicron Variant Is Highly Resistant against Antibody-Mediated Neutralization: Implications for Control of the COVID-19 Pandemic, Cell,
doi:10.1016/j.cell.2021.12.032Iuliano, Brunkard, Boehmer, Peterson, Adjei et al., Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods-United States, MMWR Morb. Mortal. Wkly. Rep,
doi:10.15585/mmwr.mm7104e4Mader, Tydykov, Glück, Bertok, Weidlich et al., Omicron's Binding to Sotrovimab, Casirivimab, Imdevimab, CR3022, and Sera from Previously Infected or Vaccinated Individuals,
doi:10.1016/j.isci.2022.104076Mazzitelli, Mengato, Sasset, Ferrari, Gardin et al., Molnupiravir and Nirmatrelvir/Ritonavir: Tolerability, Safety, and Adherence in a Retrospective Cohort Study,
doi:10.3390/v15020384Mittal, Manjunath, Ranjan, Kaushik, Kumar et al., COVID-19 Pandemic: Insights into Structure, Function, and HACE2 Receptor Recognition by SARS-CoV-2, PLoS Pathog,
doi:10.1371/journal.ppat.1008762Mohsin, Mahmud, Omicron SARS-CoV-2 Variant of Concern: A Review on Its Transmissibility, Immune Evasion, Reinfection, and Severity, Medicine,
doi:10.1097/MD.0000000000029165Piccicacco, Zeitler, Ing, Montero, Faughn et al., Real-World Effectiveness of Early Remdesivir and Sotrovimab in the Highest-Risk COVID-19 Outpatients during the Omicron Surge, J. Antimicrob. Chemother
Pinchera, Buonomo, Scotto, Carrano, Salemi et al., Solid Organ Transplant Patients With Early, Mild/Moderate SARS-CoV-2 Infection: A Single-Center Experience,
doi:10.1097/TP.0000000000004150Poliseno, Drago, Poli, Altamura, Bruno et al., Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed? BioMed,
doi:10.3390/biomed3020024Pormohammad, Ghorbani, Khatami, Farzi, Baradaran et al., Comparison of Confirmed COVID-19 with SARS and MERS Cases-Clinical Characteristics, Laboratory Findings, Radiographic Signs and Outcomes: A Systematic Review and Meta-analysis, Rev. Med,
doi:10.1002/rmv.2112Radcliffe, Palacios, Azar, Cohen, Malinis, Real-World Experience with Available, Outpatient COVID-19 Therapies in Solid Organ Transplant Recipients during the Omicron Surge, Am. J. Transplant,
doi:10.1111/ajt.17098Scovino, Dahab, Vieira, Freire-De-Lima, Freire-De-Lima et al., SARS-CoV-2's Variants of Concern: A Brief Characterization, Front. Immunol,
doi:10.3389/fimmu.2022.834098Superiore, Sanità, Flash Survey Iss: Il 17 Gennaio Il 95,8% Dei Campioni Positivi a Omicron
Takashita, Yamayoshi, Halfmann, Wilson, Ries et al., None, In Vitro Efficacy of Antiviral Agents against Omicron Subvariant BA,
doi:10.1056/NEJMc2211845Touret, Baronti, Bouzidi, De Lamballerie, In Vitro Evaluation of Therapeutic Antibodies against a SARS-CoV-2 Omicron B.1.1.529 Isolate, Sci. Rep,
doi:10.1038/s41598-022-08559-5Vaira, De Vito, Deiana, Pes, Giovanditto et al., Correlations between IL-6 Serum Level and Olfactory Dysfunction Severity in COVID-19 Patients: A Preliminary Study, Eur. Arch. Otorhinolaryngol,
doi:10.1007/s00405-021-06868-5Vaira, De Vito, Deiana, Pes, Giovanditto et al., Systemic Inflammatory Markers and Psychophysical Olfactory Scores in Coronavirus Disease 2019 Patients: Is There Any Correlation?, J. Laryngol. Otol,
doi:10.1017/S0022215121001651Vaira, Deiana, Fois, Pirina, Madeddu et al., Objective Evaluation of Anosmia and Ageusia in COVID-19 Patients: A Single-center Experience on 72 Cases, Head Neck,
doi:10.1002/hed.26204Vanblargan, Errico, Halfmann, Zost, Crowe et al., An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Therapeutic Monoclonal Antibodies, Nat. Med,
doi:10.1038/s41591-021-01678-yWoo, Brehm, Fischer, Heyer, Wichmann et al., Sotrovimab in Hospitalized Patients with SARS-CoV-2 Omicron Variant Infection: A Propensity Score-Matched Retrospective Cohort Study,
doi:10.1128/spectrum.04103-22Zinellu, De Vito, Scano, Paliogiannis, Fiore et al., The PaO 2 /FiO 2 Ratio on Admission Is Independently Associated with Prolonged Hospitalization in COVID-19 Patients, J. Infect. Dev. Ctries,
doi:10.3855/jidc.13288DOI record:
{
"DOI": "10.3390/v15081757",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v15081757",
"abstract": "<jats:p>(1) Introduction: Since May 2021, sotrovimab has been available in Italy for early treatment of SARS-CoV-2 infection and to prevent disease progression. However, some in vitro studies have questioned its efficacy on Omicron variants. Therefore, we aim to further investigate the efficacy of sotrovimab in real-life settings. (2) Methods: We conducted a retrospective study collecting medical records of people with SARS-CoV-2 infection evaluated in the infectious diseases units of Sassari, Foggia, and Bari, Italy. We included people with SARS-CoV-2 infection treated with sotrovimab and people who did not receive any treatment in 2022. The primary study outcome was to evaluate the efficacy of sotrovimab in reducing disease progression (defined as the necessity of starting oxygen supplementation) and COVID-19-related death. The secondary outcome was to evaluate the safety of sotrovimab. (3) Results: We included 689 people; of them, 341 were treated with sotrovimab, while 348 did not receive any treatment. Overall, we registered 161 (23.4%) disease progressions and 65 (9.4%) deaths, with a significant difference between treated and not-treated people (p < 0.001). In the multivariate logistic regression, increasing age [OR for ten years increasing age 1.23 (95%CI 1.04–1.45)] was associated with a higher risk of disease progression. In addition, cardiovascular disease [OR 1.69 (1.01–2.80), fever [OR 3.88 (95%CI 2.35–6.38)], and dyspnea [OR 7.24 (95%CI 4.17–12.58)] were associated with an increased risk of disease progression. In contrast, vaccination [OR 0.21 (95%CI 0.12–0.37)] and sotrovimab administration [OR 0.05 (95%CI 0.02–0.11)] were associated with a lower risk of developing severe COVID-19. Regarding mortality, people with older age [OR for ten years increasing age 1.36 (95%CI 1.09–1.69)] had a higher risk of death. In addition, in the multivariate analysis, cardiovascular disease lost statistical significance, while people on chemotherapy for haematological cancer [OR 4.07 (95%CI 1.45–11.4)] and those with dyspnea at diagnosis [OR 3.63 (95%CI 2.02–6.50)] had an increased risk of death. In contrast, vaccination [OR 0.37 (95%CI 0.20–0.68)] and sotrovimab treatment [OR 0.16 (95%CI 0.06–0.42)] were associated with lower risk. Only two adverse events were reported; one person complained of diarrhoea a few hours after sotrovimab administration, and one had an allergic reaction with cutaneous rash and itching. (4) Conclusions: Our study showed that sotrovimab treatment was associated with a reduction of the risk of disease progression and death in SARS-CoV-2-infected people, 70% of whom were over 65 years and a with high vaccination rate, with excellent safety. Therefore, our results reinforce the evidence about the efficacy and safety of sotrovimab during the Omicron era in a real-world setting.</jats:p>",
"alternative-id": [
"v15081757"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8265-5400",
"affiliation": [
{
"name": "Unit of Infectious Diseases, Department of Medicine, Surgery, and Pharmacy, University of Sassari, 07100 Sassari, Italy"
}
],
"authenticated-orcid": false,
"family": "De Vito",
"given": "Andrea",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8959-1403",
"affiliation": [
{
"name": "Unit of Infectious Diseases, Department of Medicine, Surgery, and Pharmacy, University of Sassari, 07100 Sassari, Italy"
}
],
"authenticated-orcid": false,
"family": "Colpani",
"given": "Agnese",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9896-489X",
"affiliation": [
{
"name": "S.C. Malattie Infettive, Dipartimento di Medicina Clinica e Sperimentale, University of Foggia, 71100 Foggia, Italy"
}
],
"authenticated-orcid": false,
"family": "Poliseno",
"given": "Mariacristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinic of Infectious Diseases, Department of Precision and Regenerative Medicine and Ionian Area—(DiMePRe-J), University of Bari “Aldo Moro”, Piazza Giulio Cesare n. 11, 70100 Bari, Italy"
}
],
"family": "Diella",
"given": "Lucia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "S.C. Malattie Infettive, Dipartimento di Medicina Clinica e Sperimentale, University of Foggia, 71100 Foggia, Italy"
}
],
"family": "Ieva",
"given": "Francesco Rosario Paolo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinic of Infectious Diseases, Department of Precision and Regenerative Medicine and Ionian Area—(DiMePRe-J), University of Bari “Aldo Moro”, Piazza Giulio Cesare n. 11, 70100 Bari, Italy"
}
],
"family": "Belati",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "S.C. Malattie Infettive, Dipartimento di Medicina Clinica e Sperimentale, University of Foggia, 71100 Foggia, Italy"
}
],
"family": "Papale",
"given": "Roberto",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7291-8687",
"affiliation": [
{
"name": "Unit of Infectious Diseases, Department of Medicine, Surgery, and Pharmacy, University of Sassari, 07100 Sassari, Italy"
}
],
"authenticated-orcid": false,
"family": "Babudieri",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinic of Infectious Diseases, Department of Precision and Regenerative Medicine and Ionian Area—(DiMePRe-J), University of Bari “Aldo Moro”, Piazza Giulio Cesare n. 11, 70100 Bari, Italy"
}
],
"family": "De Santis",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinic of Infectious Diseases, Department of Precision and Regenerative Medicine and Ionian Area—(DiMePRe-J), University of Bari “Aldo Moro”, Piazza Giulio Cesare n. 11, 70100 Bari, Italy"
}
],
"family": "Saracino",
"given": "Annalisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4729-7548",
"affiliation": [
{
"name": "S.C. Malattie Infettive, Dipartimento di Medicina Clinica e Sperimentale, University of Foggia, 71100 Foggia, Italy"
}
],
"authenticated-orcid": false,
"family": "Lo Caputo",
"given": "Sergio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6099-2273",
"affiliation": [
{
"name": "Unit of Infectious Diseases, Department of Medicine, Surgery, and Pharmacy, University of Sassari, 07100 Sassari, Italy"
}
],
"authenticated-orcid": false,
"family": "Madeddu",
"given": "Giordano",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T14:15:48Z",
"timestamp": 1692281748000
},
"deposited": {
"date-parts": [
[
2023,
8,
19
]
],
"date-time": "2023-08-19T04:13:41Z",
"timestamp": 1692418421000
},
"indexed": {
"date-parts": [
[
2023,
8,
20
]
],
"date-time": "2023-08-20T05:05:54Z",
"timestamp": 1692507954938
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
8,
17
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T00:00:00Z",
"timestamp": 1692230400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/15/8/1757/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1757",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
8,
17
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
17
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1080/22221751.2020.1719902",
"article-title": "Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_1",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"article-title": "SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis",
"author": "Cevik",
"doi-asserted-by": "crossref",
"first-page": "e13",
"journal-title": "Lancet Microbe",
"key": "ref_2",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2112",
"article-title": "Comparison of Confirmed COVID-19 with SARS and MERS Cases—Clinical Characteristics, Laboratory Findings, Radiographic Signs and Outcomes: A Systematic Review and Meta-analysis",
"author": "Pormohammad",
"doi-asserted-by": "crossref",
"first-page": "e2112",
"journal-title": "Rev. Med. Virol.",
"key": "ref_3",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/s41579-022-00713-0",
"article-title": "SARS-CoV-2 Pathogenesis",
"author": "Lamers",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_4",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.20944/preprints202005.0260.v2",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Mittal, A., Manjunath, K., Ranjan, R.K., Kaushik, S., Kumar, S., and Verma, V. (2020). COVID-19 Pandemic: Insights into Structure, Function, and HACE2 Receptor Recognition by SARS-CoV-2. PLoS Pathog., 16."
},
{
"DOI": "10.1371/journal.pone.0248009",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "De Vito, A., Fiore, V., Princic, E., Geremia, N., Panu Napodano, C.M., Muredda, A.A., Maida, I., Madeddu, G., and Babudieri, S. (2021). Predictors of Infection, Symptoms Development, and Mortality in People with SARS-CoV-2 Living in Retirement Nursing Homes. PLoS ONE, 16."
},
{
"DOI": "10.1002/hed.26204",
"article-title": "Objective Evaluation of Anosmia and Ageusia in COVID-19 Patients: A Single-center Experience on 72 Cases",
"author": "Vaira",
"doi-asserted-by": "crossref",
"first-page": "1252",
"journal-title": "Head Neck",
"key": "ref_7",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1097/IPC.0000000000000952",
"article-title": "A Case of Vasculitis-Like Skin Eruption Associated With COVID-19",
"author": "Geremia",
"doi-asserted-by": "crossref",
"first-page": "e30",
"journal-title": "Infect. Dis. Clin. Pract.",
"key": "ref_8",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.3582819",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Grant, M.C., Geoghegan, L., Arbyn, M., Mohammed, Z., McGuinness, L., Clarke, E.L., and Wade, R.G. (2020). The Prevalence of Symptoms in 24,410 Adults Infected by the Novel Coronavirus (SARS-CoV-2; COVID-19): A Systematic Review and Meta-Analysis of 148 Studies from 9 Countries. PLoS ONE, 15."
},
{
"DOI": "10.1017/S0022215121001651",
"article-title": "Systemic Inflammatory Markers and Psychophysical Olfactory Scores in Coronavirus Disease 2019 Patients: Is There Any Correlation?",
"author": "Vaira",
"doi-asserted-by": "crossref",
"first-page": "723",
"journal-title": "J. Laryngol. Otol.",
"key": "ref_10",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1007/s00405-021-06868-5",
"article-title": "Correlations between IL-6 Serum Level and Olfactory Dysfunction Severity in COVID-19 Patients: A Preliminary Study",
"author": "Vaira",
"doi-asserted-by": "crossref",
"first-page": "811",
"journal-title": "Eur. Arch. Otorhinolaryngol",
"key": "ref_11",
"volume": "279",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00799-6",
"article-title": "Characterisation of In-Hospital Complications Associated with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol UK: A Prospective, Multicentre Cohort Study",
"author": "Drake",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Lancet",
"key": "ref_12",
"volume": "398",
"year": "2021"
},
{
"article-title": "Clinical Features, Laboratory Findings and Predictors of Death in Hospitalized Patients with COVID-19 in Sardinia, Italy",
"author": "Geremia",
"first-page": "7861",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_13",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.3855/jidc.13288",
"article-title": "The PaO2/FiO2 Ratio on Admission Is Independently Associated with Prolonged Hospitalization in COVID-19 Patients",
"author": "Zinellu",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "J. Infect. Dev. Ctries.",
"key": "ref_14",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1503/cmaj.1095949",
"article-title": "Alpha, Beta, Delta, Gamma: What’s Important to Know about SARS-CoV-2 Variants of Concern?",
"author": "Duong",
"doi-asserted-by": "crossref",
"first-page": "E1059",
"journal-title": "CMAJ Can. Med. Assoc. J.",
"key": "ref_15",
"volume": "193",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.834098",
"article-title": "SARS-CoV-2’s Variants of Concern: A Brief Characterization",
"author": "Scovino",
"doi-asserted-by": "crossref",
"first-page": "3541",
"journal-title": "Front. Immunol.",
"key": "ref_16",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00056-3",
"article-title": "Omicron Severity: Milder but Not Mild",
"author": "Nealon",
"doi-asserted-by": "crossref",
"first-page": "412",
"journal-title": "Lancet",
"key": "ref_17",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1038/s41577-022-00720-5",
"article-title": "Estimating Disease Severity of Omicron and Delta SARS-CoV-2 Infections",
"author": "Sigal",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_18",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1097/MD.0000000000029165",
"article-title": "Omicron SARS-CoV-2 Variant of Concern: A Review on Its Transmissibility, Immune Evasion, Reinfection, and Severity",
"author": "Mohsin",
"doi-asserted-by": "crossref",
"first-page": "E29165",
"journal-title": "Medicine",
"key": "ref_19",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1016/j.isci.2022.104076",
"article-title": "Omicron’s Binding to Sotrovimab, Casirivimab, Imdevimab, CR3022, and Sera from Previously Infected or Vaccinated Individuals",
"author": "Mader",
"doi-asserted-by": "crossref",
"first-page": "104076",
"journal-title": "iScience",
"key": "ref_20",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28011",
"article-title": "Safety and Efficacy of Molnupiravir in SARS-CoV-2 Infected Patients: A Real-Life Experience",
"author": "Colpani",
"doi-asserted-by": "crossref",
"first-page": "5582",
"journal-title": "J. Med. Virol.",
"key": "ref_21",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3390/v15010071",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "De Vito, A., Colpani, A., Saderi, L., Puci, M., Zauli, B., Fiore, V., Fois, M., Meloni, M.C., Bitti, A., and Di Castri, C. (2023). Impact of Early SARS-CoV-2 Antiviral Therapy on Disease Progression. Viruses, 15."
},
{
"DOI": "10.3390/v15020384",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Mazzitelli, M., Mengato, D., Sasset, L., Ferrari, A., Gardin, S., Scaglione, V., Bonadiman, N., Calandrino, L., Cavinato, S., and Trivellato, S. (2023). Molnupiravir and Nirmatrelvir/Ritonavir: Tolerability, Safety, and Adherence in a Retrospective Cohort Study. Viruses, 15."
},
{
"DOI": "10.1016/S2666-5247(21)00069-0",
"article-title": "COVID-19 Vaccine Efficacy and Effectiveness—The Elephant (Not) in the Room",
"author": "Olliaro",
"doi-asserted-by": "crossref",
"first-page": "e279",
"journal-title": "Lancet Microbe",
"key": "ref_24",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.3390/vaccines11050896",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "De Vito, A., Colpani, A., Trunfio, M., Fiore, V., Moi, G., Fois, M., Leoni, N., Ruiu, S., Babudieri, S., and Calcagno, A. (2023). Living with HIV and Getting Vaccinated: A Narrative Review. Vaccines, 11."
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Therapeutic Monoclonal Antibodies",
"author": "VanBlargan",
"doi-asserted-by": "crossref",
"first-page": "490",
"journal-title": "Nat. Med.",
"key": "ref_26",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-08559-5",
"article-title": "In Vitro Evaluation of Therapeutic Antibodies against a SARS-CoV-2 Omicron B.1.1.529 Isolate",
"author": "Touret",
"doi-asserted-by": "crossref",
"first-page": "4683",
"journal-title": "Sci. Rep.",
"key": "ref_27",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2211845",
"article-title": "In Vitro Efficacy of Antiviral Agents against Omicron Subvariant BA.4.6",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "2094",
"journal-title": "N. Engl. J. Med.",
"key": "ref_28",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27588",
"article-title": "Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines",
"author": "Araf",
"doi-asserted-by": "crossref",
"first-page": "1825",
"journal-title": "J. Med. Virol.",
"key": "ref_29",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27697",
"article-title": "Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review",
"author": "Chenchula",
"doi-asserted-by": "crossref",
"first-page": "2969",
"journal-title": "J. Med. Virol.",
"key": "ref_30",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00514-1",
"article-title": "Omicron: Fewer Adverse Outcomes Come with New Dangers",
"author": "Post",
"doi-asserted-by": "crossref",
"first-page": "1280",
"journal-title": "Lancet",
"key": "ref_31",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7104e4",
"article-title": "Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods-United States, December 2020–January 2022",
"author": "Iuliano",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "ref_32",
"volume": "71",
"year": "2022"
},
{
"key": "ref_33",
"unstructured": "Ministero Della Salute—Istituto Superiore di Sanità (2022). Aggiornamento Casi COVID-19—Dati AggregatiQuotidianiRegioni/PPAA, Istituto Superiore di Sanità."
},
{
"key": "ref_34",
"unstructured": "Istituto Superiore di Sanità (2023, August 11). Comunicato Stampa N°08/2022-Covid-19, Flash Survey Iss: Il 17 Gennaio Il 95,8% Dei Campioni Positivi a Omicron. Available online: https://www.iss.it/web/guest/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/comunicato%C2%A0stampa-n%C2%B008-2022-covid-19-flash-survey-iss-il-17-gennaio-il-95-8-dei-campioni-positivi-a-omicron?_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId=6608164&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_redirect=https%3A%2F%2Fwww.iss.it%2Fweb%2Fguest%2Fcov19-cosa-fa-iss-varianti%3Fp_p_id%3Dcom_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId%3D6608164%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur%3D0%26p_r_p_resetCur%3Dfalse."
},
{
"DOI": "10.3390/biomed3020024",
"article-title": "Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed?",
"author": "Poliseno",
"doi-asserted-by": "crossref",
"first-page": "272",
"journal-title": "BioMed",
"key": "ref_35",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"article-title": "The Omicron Variant Is Highly Resistant against Antibody-Mediated Neutralization: Implications for Control of the COVID-19 Pandemic",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Cell",
"key": "ref_36",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N. Engl. J. Med.",
"key": "ref_37",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkac256",
"article-title": "Real-World Effectiveness of Early Remdesivir and Sotrovimab in the Highest-Risk COVID-19 Outpatients during the Omicron Surge",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"first-page": "2693",
"journal-title": "J. Antimicrob. Chemother.",
"key": "ref_38",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1007/s40121-022-00755-0",
"article-title": "Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "607",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_39",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1016/j.kint.2022.04.003",
"article-title": "Early Treatment with Sotrovimab Monoclonal Antibody in Kidney Transplant Recipients with Omicron Infection",
"author": "Chavarot",
"doi-asserted-by": "crossref",
"first-page": "1290",
"journal-title": "Kidney Int.",
"key": "ref_40",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17098",
"article-title": "Real-World Experience with Available, Outpatient COVID-19 Therapies in Solid Organ Transplant Recipients during the Omicron Surge",
"author": "Radcliffe",
"doi-asserted-by": "crossref",
"first-page": "2458",
"journal-title": "Am. J. Transplant.",
"key": "ref_41",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004150",
"article-title": "Sotrovimab in Solid Organ Transplant Patients With Early, Mild/Moderate SARS-CoV-2 Infection: A Single-Center Experience",
"author": "Pinchera",
"doi-asserted-by": "crossref",
"first-page": "e345",
"journal-title": "Transplantation",
"key": "ref_42",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2023.02.012",
"article-title": "Real-World Effectiveness of Molnupiravir, Nirmatrelvir-Ritonavir, and Sotrovimab on Preventing Hospital Admission among Higher-Risk Patients with COVID-19 in Wales: A Retrospective Cohort Study",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "352",
"journal-title": "J. Infect.",
"key": "ref_43",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1128/spectrum.04103-22",
"article-title": "Sotrovimab in Hospitalized Patients with SARS-CoV-2 Omicron Variant Infection: A Propensity Score-Matched Retrospective Cohort Study",
"author": "Woo",
"doi-asserted-by": "crossref",
"first-page": "e0410322",
"journal-title": "Microbiol. Spectr.",
"key": "ref_44",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiac206",
"article-title": "Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "2129",
"journal-title": "J. Infect. Dis.",
"key": "ref_45",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.1016/j.lanepe.2023.100694",
"article-title": "Shedding New Light on COVID-19 Therapeutics during the Omicron Era: A Deeper Dive into Real-World Data",
"author": "Colpani",
"doi-asserted-by": "crossref",
"first-page": "100694",
"journal-title": "Lancet Reg. Health Eur.",
"key": "ref_46",
"volume": "31",
"year": "2023"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/15/8/1757"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study",
"type": "journal-article",
"volume": "15"
}