Real world Effectiveness of Sotrovimab in Preventing COVID-19–related Hospitalisation or Death in Patients Infected with Omicron BA.2
Alwaleed Behzad, Aamal Mohamed, Ahmed Ali, Sara Niinuma, Alexandra E Butler, Manaf Alqahtani
Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.11.029
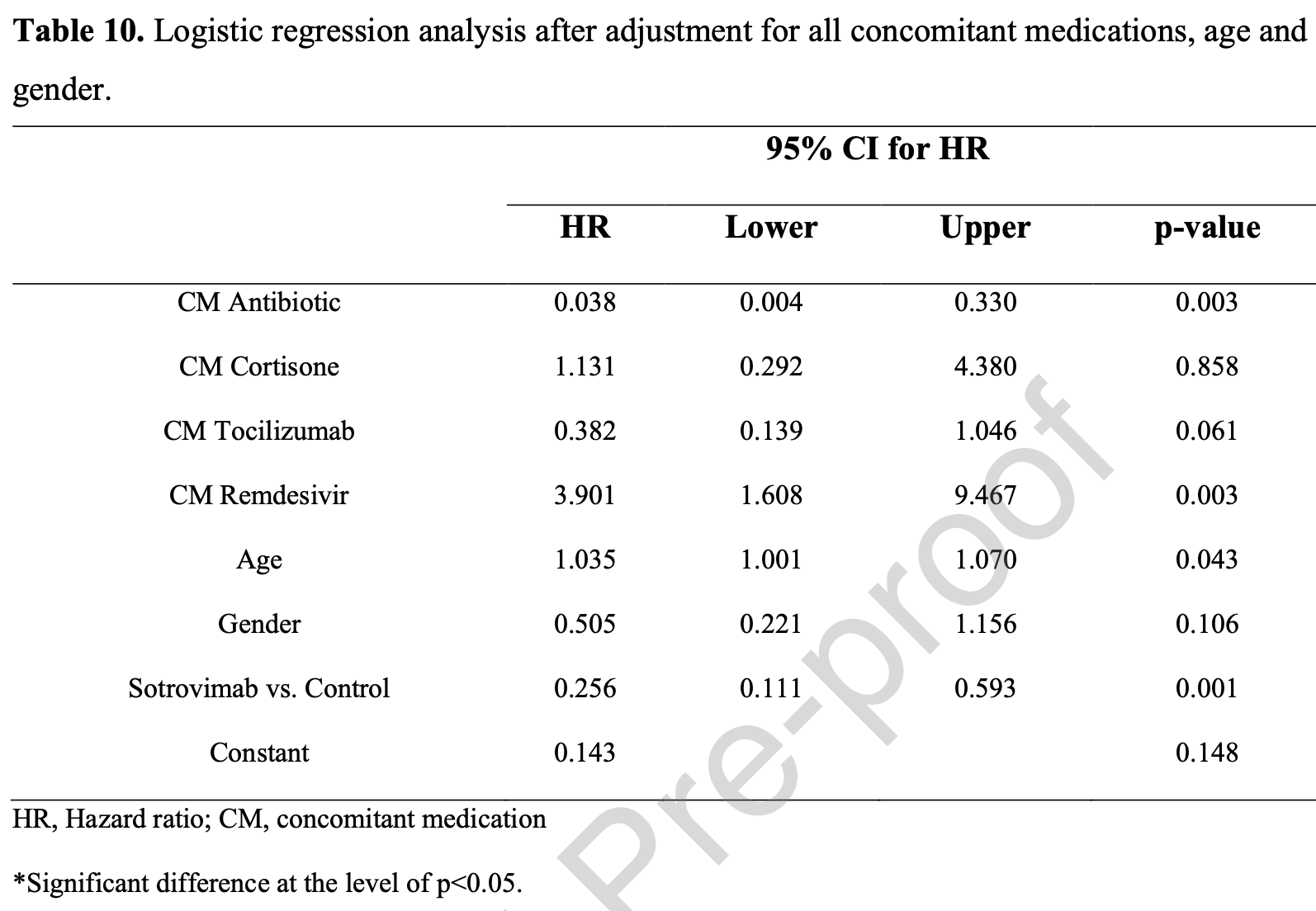
Background. Laboratory-based evidence indicates that neutralization of the BA.2 (Omicron) variant by sotrovimab is reduced versus previous SARS-CoV-2 variants. Since there is a lack of real-world data, we investigated whether sotrovimab has reduced clinical efficacy against the BA.2 variant. Methods. We performed a prospective cohort study using real-world data from 1180 randomlyselected BA.2 variant-infected patients. Follow-up to study endpoints averaged 29 days. For mild cases (not requiring oxygen-supplementation), primary outcomes were requiring O2supplementation, intensive care unit (ICU) admission or death. For moderate-to-severe COVID-19 cases (requiring oxygen-supplementation other than mechanical ventilation), the primary outcome was ICU admission or death. Results. Patients in the sotrovimab group (n=569) and control patients (n=611) were included. Sotrovimab-treated patients versus controls had reduced risk of death (0.4% vs 6.4%, p<0.001), need for oxygen supplementation (3.5% vs 12.8%, p<0.001) and ICU admission (0.2% vs 4.9%, p<0.001). The adjusted-odds ratio for developing any of these outcomes was 0.090 (95% CI 0.049-0.165, p<0.001). Subgroup analysis of moderate-to-severe sotrovimab-treated patients versus controls revealed reduced mortality (17.7% vs 37.2%, p=0.006) and ICU admission (0.0% vs 37.2%, p<0.001). Adjusted-hazards ratio for death or ICU admission was 0.256 (95% CI 0.111-0.593, p<0.001).
Conclusion. Sotrovimab was effective in reducing COVID-19 progression risk in high-risk BA.2 variant-infected patients. This finding may alleviate concerns about its clinical efficacy.
Ethics approval and consent to participate: The study was approved by the National COVID-19 Research Committee and the Bahrain Defence Force Hospital Ethics committee (Study code: CRT-COVID2022-155, approved on January 24, 2022). The study was conducted according to the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practices guidelines (ICH-GCP E6) and local regulations. All patients enrolled in the study provided written informed consent. Consent for publication: All authors gave their consent for publication.
Conflict of interest: None of the authors have any conflict of interest to declare.
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. a Mortality is significantly higher in the moderate-to-severe cases as compared to controls. b Oxygen supplementation was significantly lower in the moderate-to-severe cases as compared to control. c ICU admission was significantly lower in the moderate-to-severe cases as compared to control. d Main outcome of any of the three above was not significantly different between the moderate-to-severe cases and the control. *Significant difference at the level of p<0.05. J o u r n a l P r e -p r o o f
References
Arora, Zhang, Krüger, Rocha, Sidarovich et al., SARS-COV-2 omicron sublineages show comparable cell entry but differential neutralization by therapeutic antibodies, Cell Host & Microbe
Bruel, Hadjadj, Maes, Planas, Seve et al., Serum neutralization of SARS-COV-2 omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nature Medicine
Cox, Peacock, Harvey, Hughes, Wright et al., SARS-COV-2 variant evasion of monoclonal antibodies based on in vitro studies, Nature Reviews Microbiology
Fiaschi, Dragoni, Schiaroli, Bergna, Rossetti et al., Efficacy of licensed monoclonal antibodies and antiviral agents against the SARS-COV-2 omicron sublineages BA.1 and BA.2, Viruses
Gliga, Luebke, Killer, Gruell, Walker et al., Rapid selection of sotrovimab escape variants in SARS-CoV-2 Omicron infected immunocompromised patients, Clin Infect Dis
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Iketani, Liu, Guo, Chan, Huang et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
J O U R N A L P R E -P R O O F 10-Zhou, Dcosta, Landau, Tada, Resistance of SARS-COV-2 omicron ba.1 and BA.2 variants to vaccine-elicited Sera and therapeutic monoclonal antibodies, Viruses
J O U R N A L P R E -P R O O F 20-Mazzotta, Lepri, Colavita, Rosati, Lalle et al., Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents, J Med Virol
Kawaoka, Uraki, Kiso, Iida, Imai et al., Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA, Res Sq
Martin-Blondel, Marcelin, Soulié, Kaisaridi, Lusivika-Nzinga et al., Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2, J Infect
Ohashi, Hishiki, Akazawa, Kim, Woo et al., Different efficacies of neutralizing antibodies and antiviral drugs on SARS-COV-2 omicron subvariants, Ba.1 and BA.2, Antiviral Research
Razonable, Tulledge-Scheitel, Hanson, Arndt, Speicher et al., Real-world clinical outcomes of Bebtelovimab and SOTROVIMAB treatment of high-risk persons with coronavirus disease 2019 during the omicron epoch, Open Forum Infectious Diseases
Self, Sandkovsky, Reilly, Vock, Gottlieb et al., Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial, Lancet Infect Dis
Shrestha, Foster, Rawlinson, Tedla, Bull, Evolution of the sars-cov-2 omicron variants BA.1 to Ba.5: Implications for immune escape and transmission, Reviews in Medical Virology
Stadler, Chai, Schlub, Cromer, Polizzotto et al., Determinants of passive antibody efficacy in SARS-CoV-2 infection, medRxiv
Touret, Baronti, Pastorino, Villarroel, Ninove et al., In vitro activity of therapeutic antibodies against SARS-COV-2 omicron Ba.1, Ba.2 and BA.5, Scientific Reports
Wilhelm, Widera, Grikscheit, Toptan, Schenk et al., Limited neutralisation of the SARS-COV-2 omicron subvariants BA.1 and BA.2 by convalescent and Vaccine Serum and monoclonal antibodies, eBioMedicine
Zaqout, Almaslamani, Chemaitelly, Hashim, Ittaman et al., Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-COV-2 in Qatar, International Journal of Infectious Diseases
DOI record:
{
"DOI": "10.1016/j.jiph.2023.11.029",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2023.11.029",
"alternative-id": [
"S1876034123004355"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real world Effectiveness of Sotrovimab in Preventing COVID-19–related Hospitalisation or Death in Patients Infected with Omicron BA.2"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2023.11.029"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"ORCID": "http://orcid.org/0009-0001-2414-8857",
"affiliation": [],
"authenticated-orcid": false,
"family": "Behzad",
"given": "Alwaleed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Aamal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niinuma",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Alexandra E",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1523-0429",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alqahtani",
"given": "Manaf",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
4
]
],
"date-time": "2023-12-04T17:08:01Z",
"timestamp": 1701709681000
},
"deposited": {
"date-parts": [
[
2023,
12,
4
]
],
"date-time": "2023-12-04T17:08:34Z",
"timestamp": 1701709714000
},
"indexed": {
"date-parts": [
[
2023,
12,
5
]
],
"date-time": "2023-12-05T00:24:14Z",
"timestamp": 1701735854453
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
30
]
],
"date-time": "2023-11-30T00:00:00Z",
"timestamp": 1701302400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123004355?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123004355?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jiph.2023.11.029_bib1",
"unstructured": "Tracking sars-COV-2 variants [Internet]. World Health Organization. World Health Organization; [cited 21 Nov2022]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants/"
},
{
"article-title": "SARS-COV-2 variant evasion of monoclonal antibodies based on in vitro studies",
"author": "Cox",
"journal-title": "Nature Reviews Microbiology",
"key": "10.1016/j.jiph.2023.11.029_bib2",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2023.11.029_bib3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2381",
"article-title": "Evolution of the sars‐cov‐2 omicron variants BA.1 to Ba.5: Implications for immune escape and transmission",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Reviews in Medical Virology",
"key": "10.1016/j.jiph.2023.11.029_bib4",
"volume": "32",
"year": "2022"
},
{
"article-title": "Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2",
"author": "Kawaoka",
"journal-title": "Res Sq",
"key": "10.1016/j.jiph.2023.11.029_bib5",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-COV-2 omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"issue": "6",
"journal-title": "Nature Medicine",
"key": "10.1016/j.jiph.2023.11.029_bib6",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"journal-title": "Nature.",
"key": "10.1016/j.jiph.2023.11.029_bib7",
"year": "2022"
},
{
"article-title": "In vitro activity of therapeutic antibodies against SARS-COV-2 omicron Ba.1, Ba.2 and BA.5",
"author": "Touret",
"issue": "1",
"journal-title": "Scientific Reports",
"key": "10.1016/j.jiph.2023.11.029_bib8",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105372",
"article-title": "Different efficacies of neutralizing antibodies and antiviral drugs on SARS-COV-2 omicron subvariants, Ba.1 and BA.2",
"author": "Ohashi",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Research",
"key": "10.1016/j.jiph.2023.11.029_bib9",
"volume": "205",
"year": "2022"
},
{
"DOI": "10.3390/v14061334",
"article-title": "Resistance of SARS-COV-2 omicron ba.1 and BA.2 variants to vaccine-elicited Sera and therapeutic monoclonal antibodies",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1334",
"issue": "6",
"journal-title": "Viruses",
"key": "10.1016/j.jiph.2023.11.029_bib10",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2022.104158",
"article-title": "Limited neutralisation of the SARS-COV-2 omicron subvariants BA.1 and BA.2 by convalescent and Vaccine Serum and monoclonal antibodies",
"author": "Wilhelm",
"doi-asserted-by": "crossref",
"journal-title": "eBioMedicine",
"key": "10.1016/j.jiph.2023.11.029_bib11",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2022.04.017",
"article-title": "SARS-COV-2 omicron sublineages show comparable cell entry but differential neutralization by therapeutic antibodies",
"author": "Arora",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "Cell Host & Microbe",
"key": "10.1016/j.jiph.2023.11.029_bib12",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.3390/v14071374",
"article-title": "Efficacy of licensed monoclonal antibodies and antiviral agents against the SARS-COV-2 omicron sublineages BA.1 and BA.2",
"author": "Fiaschi",
"doi-asserted-by": "crossref",
"first-page": "1374",
"issue": "7",
"journal-title": "Viruses.",
"key": "10.1016/j.jiph.2023.11.029_bib13",
"volume": "14",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2023.11.029_bib14",
"unstructured": "U.S. Food and Drug Administration. Fact Sheet For Healthcare Providers Emergency Use Authorization (EUA) Of Sotrovimab [Internet].2022 [Updated March 2022, Cited 20 Oct 2022]. Available from: https://www.fda.gov/media/149534/download"
},
{
"DOI": "10.1016/j.ijid.2022.09.023",
"article-title": "Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-COV-2 in Qatar",
"author": "Zaqout",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.jiph.2023.11.029_bib15",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac411",
"article-title": "Real-world clinical outcomes of Bebtelovimab and SOTROVIMAB treatment of high-risk persons with coronavirus disease 2019 during the omicron epoch",
"author": "Razonable",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "Open Forum Infectious Diseases",
"key": "10.1016/j.jiph.2023.11.029_bib16",
"volume": "9",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2023.11.029_bib17",
"unstructured": "F.D.A. Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19 [Internet]. U.S. Food and Drug Administration; 2021 [cited 2022 Nov 21]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19"
},
{
"DOI": "10.1016/j.jinf.2022.06.033",
"article-title": "Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2",
"author": "Martin-Blondel",
"doi-asserted-by": "crossref",
"first-page": "e104",
"issue": "4",
"journal-title": "J Infect",
"key": "10.1016/j.jiph.2023.11.029_bib18",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "622",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2023.11.029_bib19",
"volume": "22",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2023.11.029_bib20",
"unstructured": "Mazzotta V., Cozzi Lepri A., Colavita F., Rosati S., Lalle E., Cimaglia C., et al. Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents. J Med Virol. 10.1002/jmv.28186"
},
{
"DOI": "10.1101/2022.03.21.22272672",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jiph.2023.11.029_bib21",
"unstructured": "Stadler E., Chai K.L., Schlub T.E., Cromer D., Polizzotto M.N., Kent S.J., et al. Determinants of passive antibody efficacy in SARS-CoV-2 infection. medRxiv. 2022 Aug 4;"
},
{
"key": "10.1016/j.jiph.2023.11.029_bib22",
"unstructured": "Gliga S., Luebke N., Killer A., Gruell H., Walker A., Dilthey A.T., et al. Rapid selection of sotrovimab escape variants in SARS-CoV-2 Omicron infected immunocompromised patients. Clin Infect Dis. 10.1093/cid/ciac802"
},
{
"key": "10.1016/j.jiph.2023.11.029_bib23",
"unstructured": "NHRA. Bahrain COVID-19 national protocols. [Internet]. National Health Regulatory authority Bahrain; 2020 [cited 2020 Jan 07]. Available from: https://www.nhra.bh/Media/Announcement/MediaHandler/GenericHandler/documents/Announcements/NHRA_News_MOH%20ALERT_Bahrain%20COVID-19%20National%20Protocols_20200701.pdf"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034123004355"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "Real world Effectiveness of Sotrovimab in Preventing COVID-19–related Hospitalisation or Death in Patients Infected with Omicron BA.2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}