Comparison of safety and efficacy of convalescent plasma with fresh frozen plasma in severe covid-19 patients
Meenu Bajpai, Ashish Maheshwari, Suresh Kumar, Karan Chhabra, Pratibha Kale, Ashad Narayanan, Amita Gupta, Ekta Gupta, Nirupama Trehanpati, Reshu Agarwal, Kamini Gupta, MOJAHIDUL ISLAM Ankit Bhardwaj, Mojahidul Islam, Ravinder Singh, Pushpa Yadav, Guresh Kumar, Shiv K Sarin
Anais da Academia Brasileira de Ciências, doi:10.1590/0001-3765202220210202
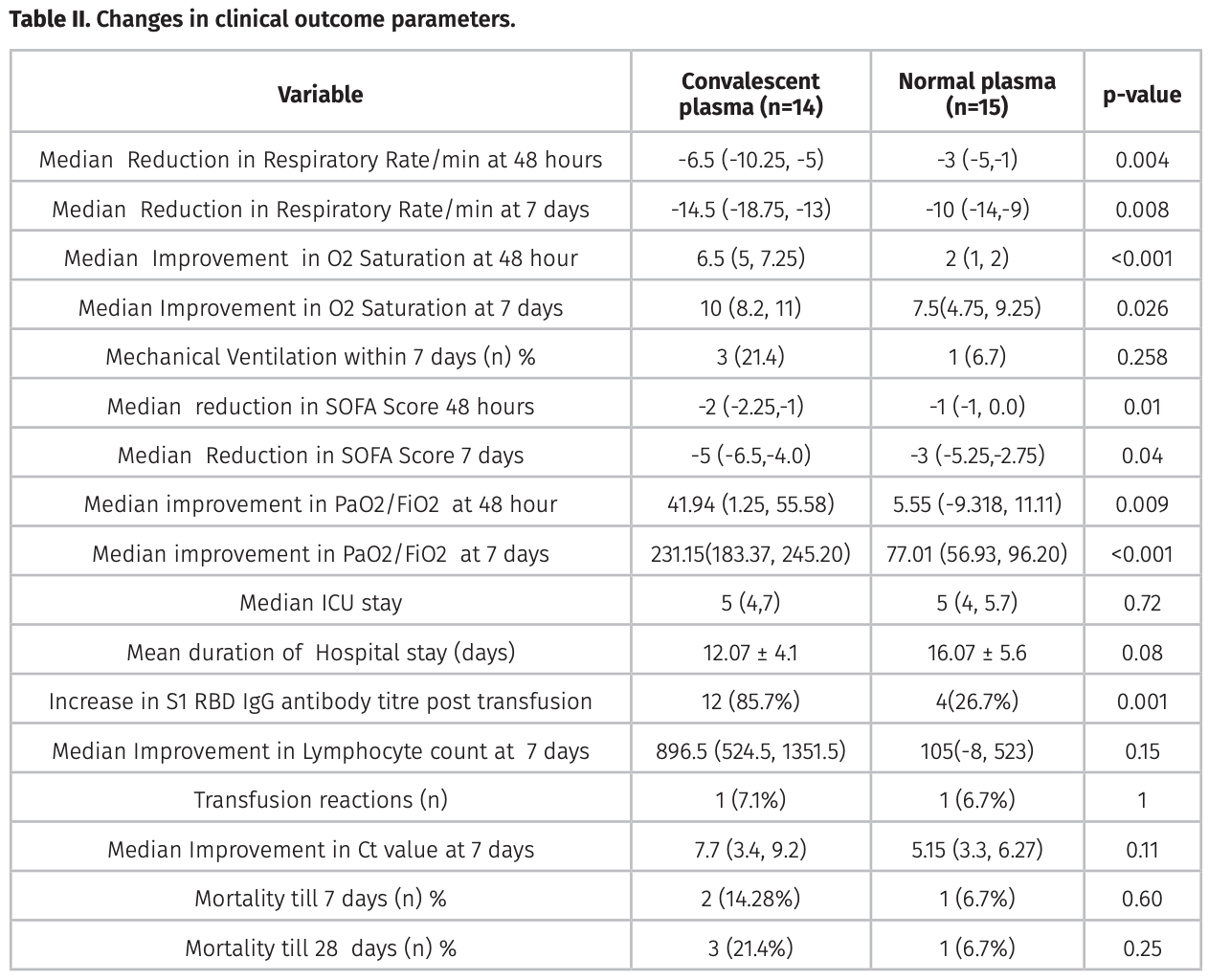
background: Role of Convalescent plasma (COPLA) to treat severe COVID-19 is under investigation. We compared effi cacy and safety of COPLA with fresh frozen plasma (FFP) in severe COVID-19 patients. Methods: One group received COPLA with standard medical care (n = 14), and another group received random donor FFP, as control with standard medical care (n = 15) in severe COVID-19 disease. Results: The proportion of patients free of ventilation at day seven were 78.5% in COPLA group, and 93.3 % in control group were not signifi cant (p= 0.258). However, improved respiratory rate, O2 saturation, SOFA score, and Ct value were observed in the COPLA group. No serious adverse events were noticed by plasma transfusion in both groups.
Author contributions 1.Study concept and design: Shiv K Sarin, Meenu Bajpai, Ashish Maheshwari, Suresh Kumar and Ekta Gupta. 2.Acquisition of data: Karan Chhabra, Ashad Narayan, Pratibha Kale, Amita Gupta, Reshu Agarwal, Kamini Gupta, Mojahidul Islam, Ravinder Singh and Pushpa Yadav. 3. Statistical analysis: Guresh Kumar, Ankit Bhardwaj. 4.Initial Drafting of the Manuscript: Suresh Kumar, Ashish Maheshwari, Pratibha Kale, Ekta Gupta, Nirupama Trehanpati, Karan Chhabra, Ankit Bhardwaj, Ashad Narayan, Amita Gupta, Reshu Agarwal, Kamini Gupta, Mojahidul Islam, Ravinder Singh and Pushpa Yadav. 5.Critical revision of manuscript done for important intellectual content: Meenu Bajpai, Guresh Kumar and Shiv K Sarin, 6.Administrative and technical support: Shiv K Sarin.
References
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentrerandomised controlled trial (PLACID trial), BMJ
Al, A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med
Al, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Chen, Xiong, Shi Y, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect Dis
De, Brito, Lc, Cf, Correia et al., The balance between the serum levels of IL-6 and IL-10 cytokines discriminates mild and severe acute pneumonia, BMC Pulm Med
Duan, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, ProcNatlAcadSci USA
Grein, Compassionate Use of Remdesivir for Patients with Severe Covid-19, N Engl J Med
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Joyner, Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo ClinProc
Li, Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomised Clinical Trial, JAMA
Libster, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Ng, Viral Load and Sequence Analysis Reveal the Symptom Severity, Diversity, and Transmission Clusters of Rhinovirus Infections, Clin Infect Dis
Recovery, Group, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Rules, Section XB and XIIB
Shen, Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma, JAMA
Straat, Mca, Jcm, Ms, Ames et al., effect of transfusion of fresh frozen plasma on parameters of
{ 'indexed': {'date-parts': [[2024, 7, 23]], 'date-time': '2024-07-23T15:04:53Z', 'timestamp': 1721747093768},
'reference-count': 19,
'publisher': 'FapUNIFESP (SciELO)',
'license': [ { 'start': { 'date-parts': [[2022, 1, 1]],
'date-time': '2022-01-01T00:00:00Z',
'timestamp': 1640995200000},
'content-version': 'vor',
'delay-in-days': 0,
'URL': 'http://creativecommons.org/licenses/by/4.0/'},
{ 'start': { 'date-parts': [[2022, 1, 1]],
'date-time': '2022-01-01T00:00:00Z',
'timestamp': 1640995200000},
'content-version': 'am',
'delay-in-days': 0,
'URL': 'http://creativecommons.org/licenses/by/4.0/'},
{ 'start': { 'date-parts': [[2022, 1, 1]],
'date-time': '2022-01-01T00:00:00Z',
'timestamp': 1640995200000},
'content-version': 'tdm',
'delay-in-days': 0,
'URL': 'http://creativecommons.org/licenses/by/4.0/'}],
'content-domain': {'domain': [], 'crossmark-restriction': False},
'published-print': {'date-parts': [[2022]]},
'DOI': '10.1590/0001-3765202220210202',
'type': 'journal-article',
'created': {'date-parts': [[2022, 9, 9]], 'date-time': '2022-09-09T20:21:28Z', 'timestamp': 1662754888000},
'source': 'Crossref',
'is-referenced-by-count': 4,
'title': 'Comparison of safety and efficacy of convalescent plasma with fresh frozen plasma in severe '
'covid-19 patients',
'prefix': '10.1590',
'author': [ { 'ORCID': 'http://orcid.org/0000-0002-4872-7845',
'authenticated-orcid': False,
'given': 'MEENU',
'family': 'BAJPAI',
'sequence': 'first',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-0716-939X',
'authenticated-orcid': False,
'given': 'ASHISH',
'family': 'MAHESHWARI',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0001-8766-6937',
'authenticated-orcid': False,
'given': 'SURESH',
'family': 'KUMAR',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0001-5710-7026',
'authenticated-orcid': False,
'given': 'KARAN',
'family': 'CHHABRA',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-5172-0193',
'authenticated-orcid': False,
'given': 'PRATIBHA',
'family': 'KALE',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0003-2531-1094',
'authenticated-orcid': False,
'given': 'ASHAD',
'family': 'NARAYANAN',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0003-1369-9853',
'authenticated-orcid': False,
'given': 'AMITA',
'family': 'GUPTA',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-5237-216X',
'authenticated-orcid': False,
'given': 'EKTA',
'family': 'GUPTA',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-6109-0033',
'authenticated-orcid': False,
'given': 'NIRUPAMA',
'family': 'TREHANPATI',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-9207-3607',
'authenticated-orcid': False,
'given': 'RESHU',
'family': 'AGARWAL',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0003-2091-1007',
'authenticated-orcid': False,
'given': 'KAMINI',
'family': 'GUPTA',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-5380-2782',
'authenticated-orcid': False,
'given': 'ANKIT',
'family': 'BHARDWAJ',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0001-7219-9686',
'authenticated-orcid': False,
'given': 'MOJAHIDUL',
'family': 'ISLAM',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0003-0603-8328',
'authenticated-orcid': False,
'given': 'RAVINDER',
'family': 'SINGH',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0001-5285-7936',
'authenticated-orcid': False,
'given': 'PUSHPA',
'family': 'YADAV',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-8864-3849',
'authenticated-orcid': False,
'given': 'GURESH',
'family': 'KUMAR',
'sequence': 'additional',
'affiliation': []},
{ 'ORCID': 'http://orcid.org/0000-0002-0544-5610',
'authenticated-orcid': False,
'given': 'SHIV K.',
'family': 'SARIN',
'sequence': 'additional',
'affiliation': []}],
'member': '530',
'reference': [ { 'key': 'ref1',
'article-title': 'PLACID Trial Collaborators',
'volume': '371',
'author': 'AGARWAL A',
'year': '2020',
'journal-title': 'BMJ'},
{ 'issue': '19',
'key': 'ref2',
'doi-asserted-by': 'crossref',
'first-page': '1787',
'DOI': '10.1056/NEJMoa2001282',
'article-title': 'A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe '
'Covid-19',
'volume': '382',
'author': 'CAO B',
'year': '2020',
'journal-title': 'N Engl J Med'},
{ 'issue': '4',
'key': 'ref3',
'doi-asserted-by': 'crossref',
'first-page': '398',
'DOI': '10.1016/S1473-3099(20)30141-9',
'article-title': 'Convalescent plasma as a potential therapy for COVID-19',
'volume': '20',
'author': 'CHEN L',
'year': '2020',
'journal-title': 'Lancet Infect Dis'},
{ 'issue': '10223',
'key': 'ref4',
'doi-asserted-by': 'crossref',
'first-page': '507',
'DOI': '10.1016/S0140-6736(20)30211-7',
'article-title': 'Epidemiological and clinical characteristics of 99 cases of 2019 novel '
'coronavirus pneumonia in Wuhan, China: a descriptive study',
'volume': '395',
'author': 'CHEN N',
'year': '2020',
'journal-title': 'Lancet'},
{ 'issue': '1',
'key': 'ref5',
'doi-asserted-by': 'crossref',
'DOI': '10.1186/s12890-016-0324-z',
'article-title': 'The balance between the serum levels of IL-6 and IL-10 cytokines '
'discriminates mild and severe acute pneumonia',
'volume': '16',
'author': 'DE BRITO RC',
'year': '2016',
'journal-title': 'BMC Pulm Med'},
{ 'key': 'ref6',
'series-title': 'Section XB and XIIB, Ministry of Health and Family Welfare Govt. of '
'India',
'author': 'DRUGS AND COSMETICS ACT AND RULES',
'year': '1940'},
{ 'issue': '17',
'key': 'ref7',
'doi-asserted-by': 'crossref',
'first-page': '9490',
'DOI': '10.1073/pnas.2004168117',
'article-title': 'Effectiveness of convalescent plasma therapy in severe COVID-19 '
'patients',
'volume': '117',
'year': '2020',
'journal-title': 'ProcNatlAcadSci USA'},
{ 'issue': '24',
'key': 'ref8',
'doi-asserted-by': 'crossref',
'first-page': '2327',
'DOI': '10.1056/NEJMoa2007016',
'article-title': 'Compassionate Use of Remdesivir for Patients with Severe Covid-19',
'volume': '382',
'year': '2020',
'journal-title': 'N Engl J Med'},
{ 'issue': '10223',
'key': 'ref9',
'doi-asserted-by': 'crossref',
'first-page': '497',
'DOI': '10.1016/S0140-6736(20)30183-5',
'article-title': 'Clinical features of patients infected with 2019 novel coronavirus in '
'Wuhan, China',
'volume': '395',
'year': '2020',
'journal-title': 'Lancet'},
{ 'key': 'ref10',
'doi-asserted-by': 'crossref',
'first-page': '1888',
'DOI': '10.1016/j.mayocp.2020.06.028',
'article-title': 'Safety update: COVID-19 convalescent plasma in 20,000 hospitalized '
'patients',
'volume': '95',
'year': '2020',
'journal-title': 'Mayo ClinProc'},
{ 'key': 'ref11',
'doi-asserted-by': 'crossref',
'first-page': '610',
'DOI': '10.1056/NEJMoa2033700',
'article-title': 'Early high-titer plasma therapy to prevent severe Covid-19 in older '
'adults',
'volume': '384',
'year': '2021',
'journal-title': 'N Engl J Med'},
{ 'issue': '5',
'key': 'ref12',
'doi-asserted-by': 'crossref',
'first-page': '460',
'DOI': '10.1001/jama.2020.10044',
'article-title': 'Effect of Convalescent Plasma Therapy on Time to Clinical Improvement '
'in Patients With Severe and Life-threatening COVID-19: A Randomised '
'Clinical Trial',
'volume': '324',
'year': '2020',
'journal-title': 'JAMA'},
{ 'issue': '2',
'key': 'ref13',
'doi-asserted-by': 'crossref',
'first-page': '261',
'DOI': '10.1093/cid/ciy063',
'article-title': 'Viral Load and Sequence Analysis Reveal the Symptom Severity, '
'Diversity, and Transmission Clusters of Rhinovirus Infections',
'volume': '67',
'year': '2018',
'journal-title': 'Clin Infect Dis'},
{ 'issue': '8',
'key': 'ref14',
'doi-asserted-by': 'crossref',
'first-page': '693',
'DOI': '10.1056/NEJMoa2021436',
'article-title': '“Dexamethasone in Hospitalized Patients with Covid-19',
'volume': '384',
'author': 'RECOVERY COLLABORATIVE GROUP',
'year': '2021',
'journal-title': 'N Engl J Med'},
{ 'issue': '16',
'key': 'ref15',
'doi-asserted-by': 'crossref',
'first-page': '1582',
'DOI': '10.1001/jama.2020.4783',
'article-title': 'Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent '
'Plasma',
'volume': '323',
'year': '2020',
'journal-title': 'JAMA'},
{ 'issue': '1',
'key': 'ref16',
'doi-asserted-by': 'crossref',
'DOI': '10.1186/s13054-015-0828-6',
'article-title': 'effect of transfusion of fresh frozen plasma on parameters of '
'endothelial condition and inflammatory status in non-bleeding '
'critically ill patients: a prospective substudy of a randomized trial',
'volume': '19',
'author': 'STRAAT M',
'year': '2015',
'journal-title': 'Crit Care'},
{ 'issue': '7',
'key': 'ref17',
'doi-asserted-by': 'crossref',
'first-page': '619',
'DOI': '10.1056/NEJMoa2031304',
'article-title': 'A randomized trial of convalescent plasma in covid-19 severe pneumonia',
'volume': '384',
'year': '2021',
'journal-title': 'N Engl J Med'},
{ 'issue': '13',
'key': 'ref18',
'doi-asserted-by': 'crossref',
'first-page': '1239',
'DOI': '10.1001/jama.2020.2648',
'article-title': 'Characteristics of and Important Lessons From the Coronavirus Disease '
'2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases '
'From the Chinese Center for Disease Control and Prevention',
'volume': '323',
'author': 'WU Z',
'year': '2020',
'journal-title': 'JAMA'},
{ 'issue': '1',
'key': 'ref19',
'article-title': 'Treatment With Convalescent Plasma for Critically Ill Patients With '
'Severe Acute Respiratory Syndrome Coronavirus 2 Infection',
'volume': '158',
'year': '2020',
'journal-title': 'Chest'}],
'container-title': 'Anais da Academia Brasileira de Ciências',
'original-title': [],
'link': [ { 'URL': 'http://www.scielo.br/scielo.php?script=sci_pdf&pid=S0001-37652022000600701&tlng=en',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2022, 10, 17]],
'date-time': '2022-10-17T13:13:37Z',
'timestamp': 1666012417000},
'score': 1,
'resource': {'primary': {'URL': 'https://www.scielo.br/j/aabc/a/sFk4vCTR69vjG9wTsKHxLwC/?lang=en#'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2022]]},
'references-count': 19,
'URL': 'http://dx.doi.org/10.1590/0001-3765202220210202',
'relation': { 'has-preprint': [ { 'id-type': 'doi',
'id': '10.1101/2020.10.25.20219337',
'asserted-by': 'object'}]},
'ISSN': ['0001-3765'],
'subject': [],
'container-title-short': 'An. Acad. Bras. Ciênc.',
'published': {'date-parts': [[2022]]}}