Toward a Radically Simple Multi‐Modal Nasal Spray for Preventing Respiratory Infections
Helna John Joseph, Helna Mary Baby, Joselyn Rojas Quintero, Devin Kenney, Yohannes A Mebratu, Eshant Bhatia, Purna Shah, Kabir Swain, Dongtak Lee, Shahdeep Kaur, Xiang‐ling Li, John Mwangi, Olivia Snapper, Remya Nair, Eli Agus, Sruthi Ranganathan, Julian Kage, Jingjing Gao, James N Luo, Anthony Yu, Dongsung Park, Florian Douam, Yohannes Tesfaigzi, Jeffrey M Karp, Nitin Joshi
Advanced Materials, doi:10.1002/adma.202406348
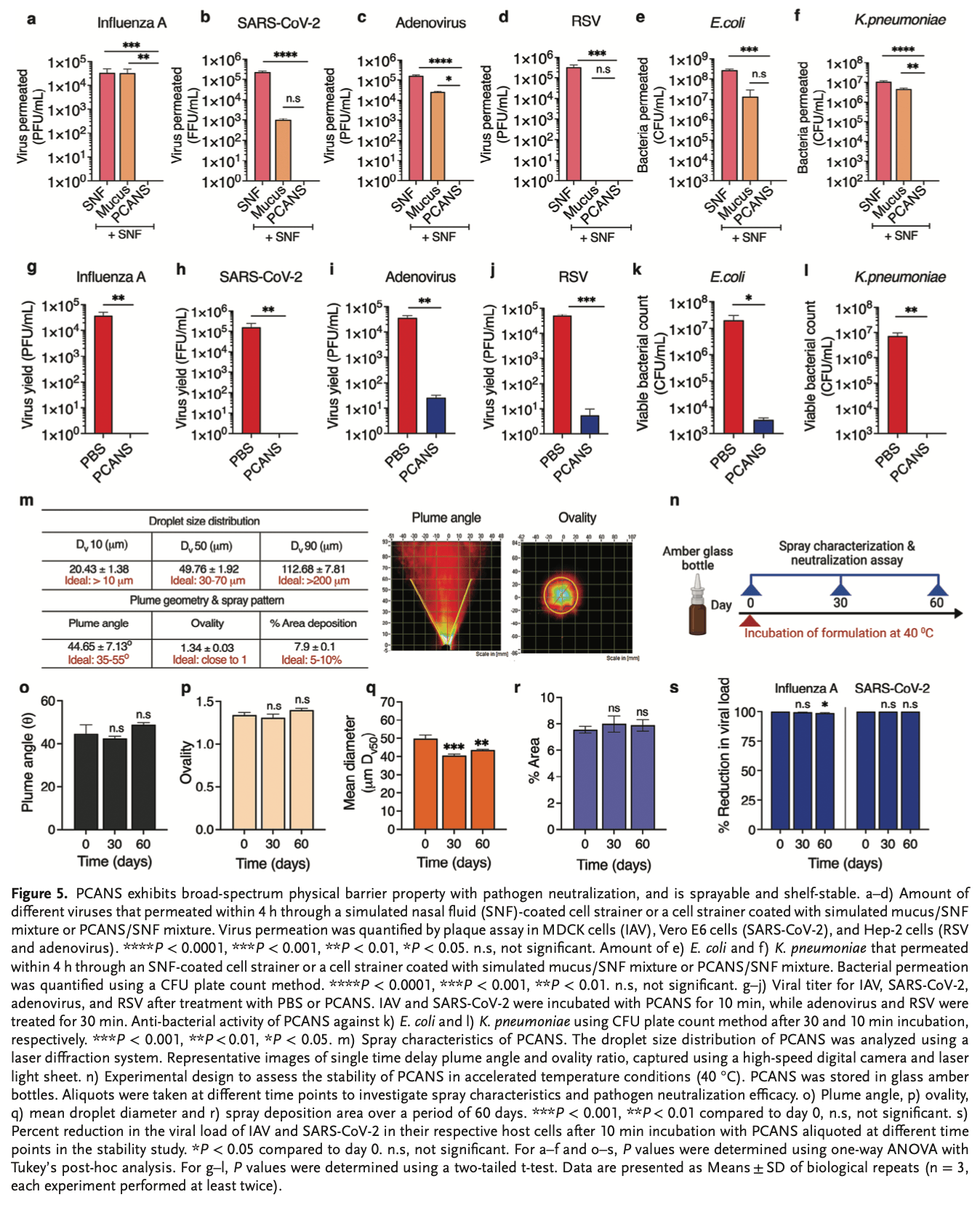
Nasal sprays for pre-exposure prophylaxis against respiratory infections show limited protection (20-70%), largely due to their single mechanism of action-either neutralizing pathogens or blocking their entry at the nasal lining, and a failure to maximize the capture of respiratory droplets, allowing them to potentially rebound and reach deeper airways. This report introduces the Pathogen Capture and Neutralizing Spray (PCANS), which utilizes a multi-modal approach to enhance efficacy. PCANS coats the nasal cavity, capturing large respiratory droplets from the air, and serving as a physical barrier against a broad spectrum of viruses and bacteria, while rapidly neutralizing them with over 99.99% effectiveness. The formulation consists of excipients identified from the FDA's Inactive Ingredient Database and Generally Recognized as Safe list to maximize efficacy for each step in the multi-modal approach. PCANS demonstrates nasal retention for up to 8 hours in mice. In a severe Influenza A mouse model, a single pre-exposure dose of PCANS leads to a >99.99% reduction in lung viral titer and ensures 100% survival, compared to 0% in the control group. PCANS suppresses pathological manifestations and offers protection for at least 4 hours. This data suggest PCANS as a promising daily-use prophylactic against respiratory infections.
Supporting Information Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest J.J., H.M.B, Y.T., and J.M.K have one pending patent based on the PCANS formulation described in this manuscript. N.J. and J.M.K are paid consultants, scientific advisory board members, and hold equity in Akita Biosciences, a company that has licensed IP generated by N.J. and J.MK. that may benefit financially if the IP was further validated. The interests of N.J. and J.MK. were reviewed and overseen by their institution in accordance with its conflict of interest policies.
Author Contributions J.J., H.M.B., and J.R.Q contributed equally to this work. J.J., H.M.B., J.R.Q., Y.T., J.M.K., N.J. performed conceptualization. J.J, H.M.B., J.R.Q., D.K., E.B., D.L., D.P. was performed data curation. J.J., H.M.B., J.R.Q., D.K., E.B., D.L., D.P. performed data analysis. J.M.K., N.J. performed funding acquisition. J.J., H.M.B., J.R.Q., Y.M., E.B., P.S., K.S., O.S., R.N., E.A., S.R., J.K. performed investigation. J.J., H.M.B., J.R.Q., D.K., S.K., X.L.L., J.M., J.G., J.N.L, A.Y., F.D. performed methodology. Y.T., J.M.K., N.J. performed project administration. F.D., Y.T., J.M.K., N.J. performed supervision. J.J., H.M.B., J.R.Q., J.M.K., N.J. performed validation. J.J., H.M.B., S.R., N.J., wrote manuscript -original draft: J.J., H.M.B., D.K., F.D., Y.T., J.M.K, N.J. edited the manuscript.
References
Ahn, Kim, Hong, Choi, Yang et al., None, J. Clin. Invest
Americo, Cotter, Earl, Liu, Moss, None, Proc Natl Acad Sci
Awasthi, Rahman, Rui, Kumar, Awasthi et al., None, BMC Pulm Med
Bailey, Wilson, None, JAMA Netw. Open
Balmforth, Swales, Silpa, Dunton, Davies et al., None, Journal of Clinical Virology
Barea, Jenkins, Gaber, Bridson, None, Int. J. Pharm
Baur, Rautenberg, Faulstich, Grau, Severin et al., None, PLoS Pathog
Belser, Gustin, Katz, Maines, Tumpey, None, Virology
Bogdan, None, Nat. Immunol
Cannon, Westover, Bleher, Sanchez-Gonzalez, Ferrer, None
Choi, Lee, Lim, Kim, Lee et al., None, Part. Fibre Toxicol
Cidem, Bradbury, Traini, Ong, None, Front. Bioeng. Biotechnol
Dirauf, Wagner, Braeuer, None, J. Supercrit. Fluids
Djupesland, Messina, Mahmoud, None, Ther Deliv
Eccleston, Bakhshaee, Hudson, Richards, None, Drug Dev. Ind. Pharm
Ehrick, Shah, Shaw, Kulkarni, Coowanitwong et al., None, Sterile Product Development
Elwinger, Pourmand, Furó, None, J. Phys. Chem. C
Excler, Saville, Berkley, Kim, None, Nat. Med
Farina, Handbook of Non-Invasive Drug Delivery Systems, Elsevier
Ferkol, Schraufnagel, None, Ann. Am. Thorac. Soc
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., None, Int. J. Gen. Med
Frank, Brown, Capriotti, Westover, Pelletier et al., None, JAMA Otolaryngol. Head Neck Surg
Frediansyah, None, Clin. Epidemiol. Glob. Health
Fröba, Große, Setz, Rauch, Auth et al., None, Int. J. Mol. Sci
Fukushi, Ito, Oka, Kitazawa, Miyoshi-Akiyama et al., None, PLoS One
Fumagalli, Ravà, Marotta, Di Lucia, Laura et al., None, Sci Immunol
Gaffar, Thacore, Buffalo Seiyoung Yun, Chemine, Ferrari Bioqualusa et al., None
Galgatte, Kumbhar, Chaudhari, None, Drug Deliv
Gizurarson, None, Acta Pharm. Nord
Gizurarson, None, Biol. Pharm. Bull
Go, Pandav, Sanchez-Gonzalez, Ferrer, None, Cureus
Gonzalez-Juarbe, Riegler, Jureka, Gilley, Brand et al., None, Cell Rep
Gowda, Ohno, Siddabasave, Chiba, Shingai et al., None, Sci. Rep
Hallworth, Westmoreland, None, J. Pharm. Pharmacol
Hamed, Fiegel, None, J. Biomed. Mater. Res A
Harding, Haasi, Chambers, Heaton, None, PLoS Pathog
He, He, Hong, Yang, Wei, None, Med. Res. Rev
Hodges, Denyer, Hanlon, Reynolds, None, J. Pharm. Pharmacol
Hossain, Hassanzadeganroudsari, Apostolopoulos, None, Expert Rev. Vaccines
Hou, Okuda, Edwards, Martinez, Asakura et al., None, Cell
Ilyushina, Khalenkov, Seiler, Forrest, Bovin et al., None, J. Virol
Ivanova, Sotirova, Gavrailov, Nikolova, Andonova, None, Pharmaceutics
Jiao, Zhang, None, Allergy Asthma Immunol. Res
Joseph, Baby, Zhao, Li, Cheung et al., None, Exploration
Joseph, None, Immuno
Kirchmajer, Steinhoff, Warren, Clark, Panhuis, None, Carbohydr. Res
Kobayashi, Suzuki, None, PLoS One
Ku, Xie, Hinton, Liu, Ye et al., None, Nature
Kulkarni, Shaw, Characteriz, None, Nasal Sprays
Leibbrandt, Meier, König-Schuster, Weinmüllner, Kalthoff et al., None, PLoS One
Lin, Lim, Xue, Yew, Owh et al., None, VIEW
Lin, Yue, Yang, Yang, Pan et al., None, Clin. Infect. Dis
Lisi, Zelikin, Chandrawati, None, Adv. Sci
Lungare, Bowen, Badhan, None, J. Pharm. Sci
Marple, Roland, Benninger, None, Otolaryngol. -Head Neck Surg
Mei, Li, Wang, Zhu, Huang et al., None, Nat. Mater
Moakes, Davies, Stamataki, Grover, None, Adv. Mater
Moreira, Da Silva, Reis, Marques, None, Polymers
Morokutti-Kurz, Fröba, Graf, Große, Grassauer et al., None, PLoS One
Patel, Verma, None, JAMA -J. Am. Med. Assoc
Pawłowski, None, AIMS Biophys
Penn, Hennessy, None, Appl. Math. Model
Pennington, Pandey, Tat, Willson, Donovan, None, Drug Dev. Ind. Pharm
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., None, Nature
Pérez-Rodríguez, Cabo, Balsa-Canto, García, None, Int. J. Mol. Sci
Rakowska, Tiddia, Faruqui, Bankier, Pei et al., None, Commun Mater
Robinson, Moakes, Grover, None, Front Med Technol
Rowe, Sheskey, Cook, Association, Fenton, Handbook of Pharmaceutical Excipients
Rugonyi, Biswas, Hall, None, Respir. Physiol. Neurobiol
Schutz, Conzelmann, Fois, Groß, Weil et al., None, Am. J .Physiol. Lung Cell Mol. Physiol
Sungnak, Huang, Bécavin, Berg, Queen et al., None, Nat. Med
Tandon, Wu, Moore, Winchester, Tu et al., None, Lancet Region. Health -Southeast Asia
Tatykhanova, Aseyev, Kudaibergenov, None, Reviews and Advances in Chemistry
Thirawong, Nunthanid, Puttipipatkhachorn, Sriamornsak, None, Eur. J. Pharm. Biopharm
Thompson, Burgess, Naleway, Tyner, Yoon et al., None, N. Engl. J. Med
Wang, Prather, Sznitman, Jimenez, Lakdawala et al., None, Science
Watson, Barnsley, Toor, Hogan, Winskill et al., None, Lancet Infect. Dis
Weiser, Ferreira, Paton, None, Nat. Rev. Microbiol
Williamson, Dennison, Greenwell, Denison-Day, Mowbray et al., None, BMJ Open
Woolley, None, J. Exp. Med
Xia, Pan, Luo, Shen, Li et al., None, Cell Discov
Xie, Muruato, Zhang, Lokugamage, Fontes-Garfias et al., None, Nat. Commun
Zarabanda, Vukkadala, Phillips, Qian, Mfuh et al., None, Laryngoscope
Zhang, None, Methods Mol. Biol